

Challenges of using real world data in research and clinical trials
Lifebit
Introduction
Patient and health information obtained outside of clinical trial settings is referred to as real world data (RWD). Analyzing RWD leads to the creation of real world evidence (RWE). The value that these kinds of data can bring to traditional clinical research and trials is growing.
However, there are still some considerable challenges surrounding the use of RWD in clinical research and trials. Compared to data gathered from randomized controlled trials (RCTs), RWD is frequently far larger and unstructured. As a result, securely gathering, storing, accessing and analyzing such large volumes of data can frequently be difficult. This blog discusses the difficulties of using RWD in clinical trials and research, and suggests potential solutions to these challenges.
Five challenges surrounding the use of real world data and real world evidence
Using RWD to decrease approval times and improve cost efficiencies in clinical trials has huge potential benefits, but existing challenges must be addressed for full realization of benefits. These include limitations in data quality and validity, a large variety of data sources, privacy and ethical considerations, and regulatory uncertainty surrounding the use of RWD.
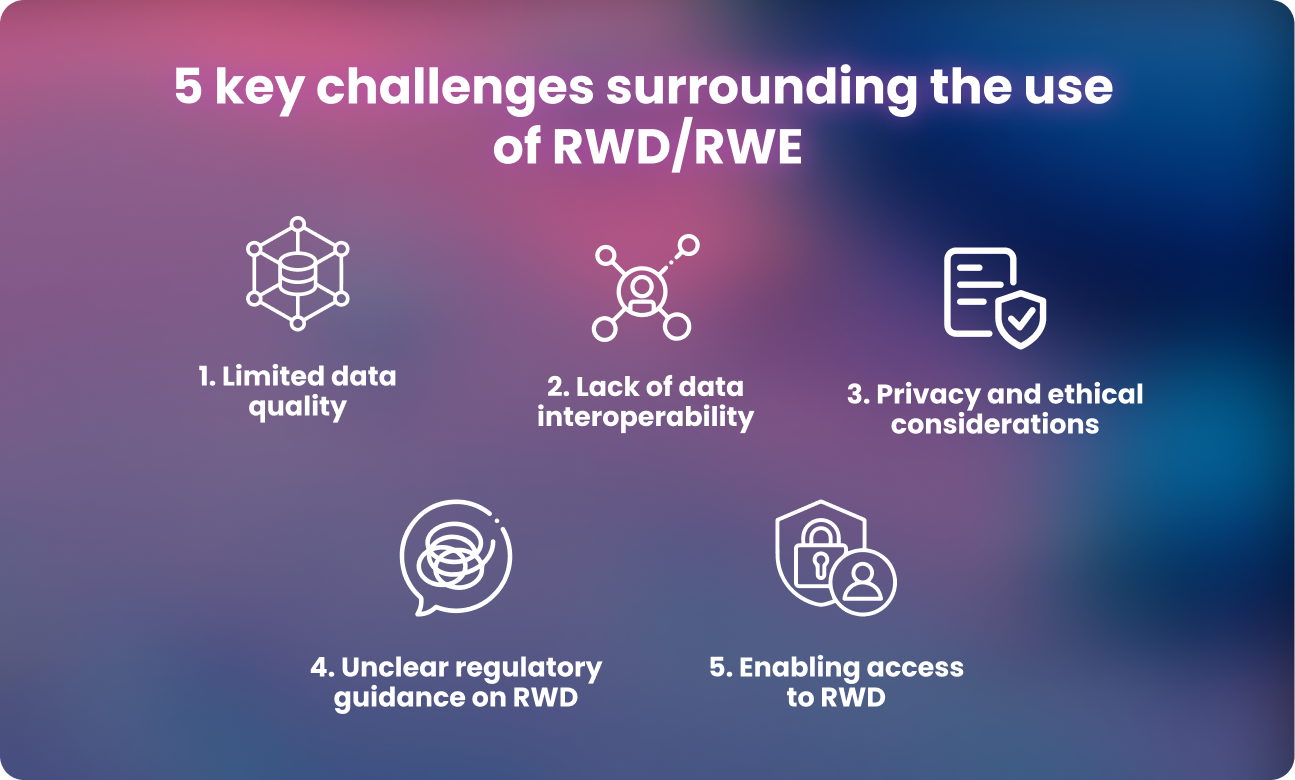
1. Limited data quality
Analyzing RWD requires sophisticated analysis platforms to mitigate confounding factors and ensure causal inference. Researchers must ensure their data is accurate, complete and adheres to rigorous study designs, transparent reporting standards, and validation processes to generate reliable evidence.
For example, a recent review analyzing the use of RWD in clinical trial approvals reported various data related challenges, including data quality issues such as missing values. This is sometimes referred to as "garbage in, garbage out" -inaccurate data will result in incorrect results and conclusions being drawn.
2. Lack of data interoperability
Ensuring the interoperability of RWD collected from a variety of sources remains a significant hurdle in its use for clinical research. Harmonizing disparate data sources is a critical consideration that requires robust governance frameworks and technological solutions to ensure RWD can be effectively integrated with other datasets.
Catch up on our recent webinar highlighting the role of data harmonization. The webinar explores the significance of high-quality, standardized data for downstream analysis to allow new scientific discoveries and enhance patient outcomes.
3. Privacy and ethical considerations
Protecting patient confidentiality and data security is crucial to prevent unauthorized access, breaches, or misuse of sensitive health information like RWD. RWD should be handled via a safe data solution that integrates best-in-class security measures, to ensure patient privacy is safeguarded and to maintain public trust in RWE generation.
Featured resource: Discover Lifebit’s approach to data security in our white paper
4. Unclear regulatory guidance on real world data and evidence
Despite the growing recognition of RWE in regulatory decision-making, there remains a need to establish clear guidelines and standards for incorporating RWD into clinical trial approvals. The establishment of a regulatory framework for RWE is essential to realize its full potential in healthcare decision-making and calls for collaborative efforts from stakeholders, including policymakers, regulatory agencies, researchers, healthcare providers, and patients.
5. Enabling secure and compliant access to real world data
Enabling access to RWD for health research involves implementing robust data governance frameworks that prioritize privacy and security while facilitating appropriate, controlled data access. Utilizing technologies like secure data catalogs can help ensure data integrity and confidentiality during data access processes.
Summary
Despite these considerable challenges, the integration of RWD into clinical trials represents a paradigm shift in evidence generation and healthcare delivery. Overcoming these obstacles requires collaboration among stakeholders, including researchers, healthcare providers, policymakers, and technology innovators.
Investments in data infrastructure, interoperability standards, and privacy-preserving technologies are crucial to unlocking the full potential of RWD in clinical research. Moreover, fostering a culture of secure data access and transparency will facilitate broader access to diverse datasets, driving innovation and improving patient outcomes.
Look out for our next blog which will go into further detail on the current guidance from the Food and Drug Administration (FDA) surrounding the use of RWD/E in clinical research and trials.
About Lifebit
Lifebit's federated technology provides secure access to deep, diverse data access from over 100 million patients. Researchers worldwide can securely connect and analyze valuable real world, clinical and genomic data in a compliant manner.
Discover our precision medicine data catalog and book a data consultation with one of our experts now.
Featured news and events
2025-03-14 15:45:18
2025-03-05 12:49:53
2025-02-27 10:00:00

2025-02-19 13:30:24
2025-02-11 08:39:49
2025-01-30 12:47:38
2025-01-28 08:00:00

2025-01-23 09:07:20

2025-01-08 13:58:41

2024-12-06 15:53:10

.png)