‘Omics: 4 Crucial Frontiers Explored
Why ‘Omics is Revolutionizing Modern Science
‘Omics is a approach in biology that studies complete sets of biological molecules to understand how living systems function. Rather than focusing on individual genes or proteins, ‘omics offers a comprehensive view of entire biological networks and their interactions.
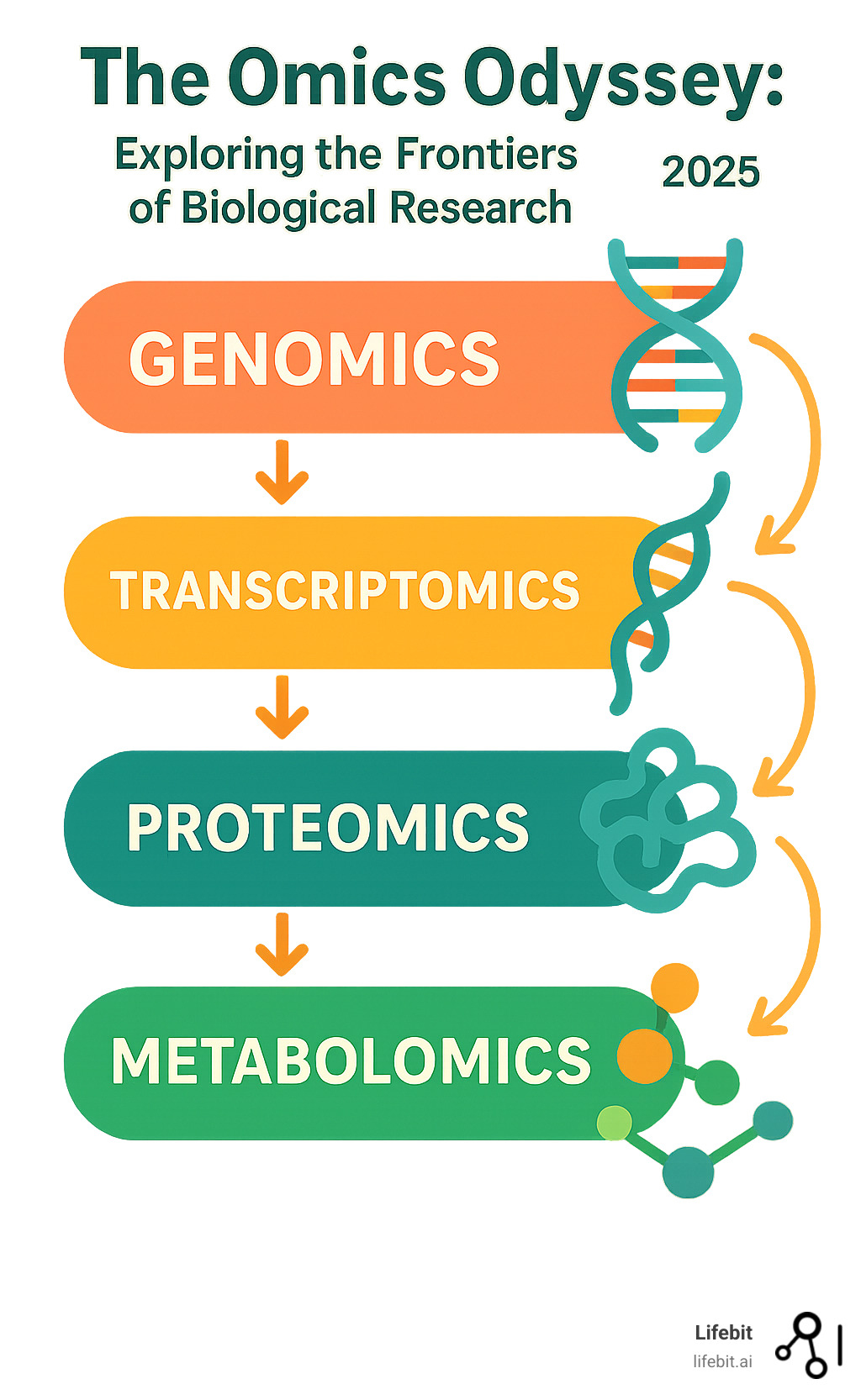
Key ‘Omics Fields:
- Genomics – Studies all DNA/genes in an organism
- Transcriptomics – Analyzes all RNA molecules and gene expression
- Proteomics – Examines all proteins and their functions
- Metabolomics – Investigates all small molecules and metabolites
- Epigenomics – Studies chemical modifications that control gene activity
The term ‘omics comes from adding the suffix “-omics” (study of) to “-ome” (totality of). This pattern began with the human genome project and has expanded to dozens of related fields.
‘Omics is changing medicine, agriculture, and environmental science by enabling researchers to:
- Find new disease biomarkers
- Develop personalized treatments
- Understand complex biological systems
- Predict drug responses
- Monitor ecosystem health
As Maria Chatzou Dunford, CEO and Co-founder of Lifebit, my 15+ years in computational biology have shown me the immense potential of ‘omics data. From building genomic tools to contributing to frameworks like Nextflow, I’ve seen how advanced data analysis is revolutionizing healthcare when ‘omics data is properly integrated and analyzed.
Decoding the Blueprint of Life: What is ‘Omics’?
Traditionally, biological research often focused on single genes or proteins. This reductionist approach, while foundational, was like trying to understand a city by studying a single building—important, but missing the intricate network of roads, communications, and interactions that make the city function. ‘Omics revolutionizes this by studying biology in its entirety. It investigates the entire collection of specific molecules or processes within an organism, comprehensively identifying, describing, and measuring them. This holistic approach allows researchers to see the whole system, generating new hypotheses and uncovering previously unseen relationships. For a deeper dive into how scientists interpret this information, you can explore scientific research on the interpretation of omics data.
The Suffixes ‘-Ome’ and ‘-Omics’ Explained
The naming of ‘omics fields provides a clue to their focus, starting with the term “genome” (the complete set of genetic material).
- ‘-Ome’: This suffix means the total collection or entire complement of a specific type of biological molecule within a cell, tissue, or organism. For example, the proteome is the complete collection of all proteins, the transcriptome is the total set of all RNA molecules, and the metabolome is the entire collection of small molecules (metabolites). It signifies a comprehensive, collective set.
- ‘-Omics’: This suffix describes the field of study that investigates the corresponding ‘-ome’. Therefore, genomics is the study of the genome, proteomics studies the proteome, and metabolomics studies the metabolome. This dual naming system, explained further in the Wiktionary definition of -omics, clearly separates the biological entity from the scientific field studying it.
The Primary Purpose of ‘Omics’ Sciences
The primary goal of ‘omics is to provide a complete, holistic understanding of biological systems by characterizing and quantifying entire sets of biological molecules. This enables a shift from a narrow, hypothesis-driven focus to a broad, findy-driven exploration.
Here’s what ‘omics aims to achieve:
- Comprehensive Characterization and Quantification: This involves identifying and measuring every molecule within a specific group—be it tens of thousands of genes, hundreds of thousands of proteins, or thousands of metabolites—in a single biological sample. This provides a high-resolution molecular snapshot of a cell, tissue, or organism at a specific point in time, offering unprecedented depth and scale.
- Understanding Structure, Function, and Dynamics: By mapping these vast molecular landscapes, ‘omics helps us understand not just the components but also their interactions, their functional roles, and how they change over time or in response to stimuli like disease, medication, or environmental factors. This dynamic view is crucial, as the state of a cell is constantly in flux.
- Unbiased Findy and Hypothesis Generation: Unlike traditional research that tests a pre-existing hypothesis (e.g., “Does gene X cause disease Y?”), ‘omics is often findy-driven. It generates massive datasets that can reveal unexpected patterns, correlations, and causal relationships, leading to novel hypotheses that would not have been conceived otherwise. For example, genomic studies unexpectedly linked the PCSK9 gene to cholesterol levels, leading to a powerful new class of cholesterol-lowering drugs.
- Revealing Biological Complexity through Systems Biology: Life is incredibly complex, governed by intricate networks of interacting molecules. ‘Omics provides the tools to map and analyze these networks, a field known as systems biology. Instead of a linear path from gene to protein to function, systems biology reveals a web of feedback loops, crosstalk, and emergent properties that define health, disease, and environmental responses.
By moving beyond reductionist analysis, ‘omics provides a systems-level understanding of biology, generating and making sense of data to open up the secrets of life.
A Tour of the Major ‘Omics’ Fields
The world of ‘omics is a journey through different levels of biological information, from the static blueprint of our DNA to the dynamic chemical reactions that power our cells. Each field provides a specialized lens to view a vital part of life’s intricate story, and together they paint a detailed picture of how living things work.
Let’s explore some of its most important branches:
| Omics Field | Molecule Studied | Key Technology | Primary Research Question |
|---|---|---|---|
| Genomics | DNA | Next-Generation Sequencing (NGS) | What is the complete genetic makeup, and how do variations influence traits/disease? |
| Epigenomics | Epigenetic marks (e.g., DNA methylation) | Bisulfite Sequencing, ChIP-Seq | How are genes regulated without changing the DNA sequence itself? |
| Transcriptomics | RNA (mRNA, non-coding RNA) | RNA Sequencing (RNA-Seq) | Which genes are active, and how do their expression levels change? |
| Proteomics | Proteins and their modifications | Mass Spectrometry (MS) | What proteins are present, what are their functions, and how do they interact? |
| Metabolomics | Metabolites (small molecules) | NMR Spectroscopy, LC-MS | What chemical processes are active, and how do they reflect cellular state? |
Genomics: The Foundation
Genomics is the study of the genome, the complete instruction manual for an organism written in DNA. This field allows scientists to sequence entire genomes to identify genetic variations—the differences in our DNA that make each of us unique. These variations range from single nucleotide polymorphisms (SNPs), where a single DNA letter is changed, to larger insertions, deletions, and copy number variations (CNVs). While whole-genome sequencing (WGS) reads the entire genetic code, techniques like whole-exome sequencing (WES) focus on the protein-coding regions. Landmark initiatives like the Human Genome Project, and more recent large-scale efforts such as the UK Biobank and the All of Us Research Program, have created vast genomic databases. These resources are foundational for personalized medicine, where treatments are custom to an individual’s genetic blueprint to predict disease risk and drug response.
Epigenomics: The Regulatory Layer
While genomics tells us what the genetic code is, epigenomics tells us how that code is used. It is the study of chemical modifications to DNA and its associated proteins that regulate gene activity without altering the underlying DNA sequence. These epigenetic marks, such as DNA methylation and histone modification, act like switches, turning genes on or off. For example, adding a methyl group to DNA often silences a gene. These patterns are crucial for normal development, allowing cells with the same DNA to differentiate into various types (e.g., a neuron vs. a skin cell). They are also dynamic and can be influenced by environmental factors. Aberrant epigenetic patterns are a hallmark of many diseases, including cancer, making epigenomics a vital field for understanding disease mechanisms and developing new therapies.
Transcriptomics: The Expression Layer
If the genome is the master manual, the transcriptome is the set of instructions actively being copied and used at any given moment. Transcriptomics studies all RNA molecules in a cell, which primarily act as messengers carrying instructions from DNA to the cell’s protein-building machinery. By measuring gene expression with techniques like RNA Sequencing (RNA-Seq), we can see which genes are “on” or “off” and at what level. This is critical for comparing different conditions, such as healthy versus cancerous tissue, through a process called differential expression analysis. This field also explores non-coding RNA (e.g., microRNAs and long non-coding RNAs), which don’t code for proteins but play key regulatory roles. While genomics tells us what could happen, transcriptomics reveals what is happening, providing a dynamic link between our genetic code and cellular function.
Proteomics: The Functional Machinery
Proteins perform most of the work in our cells, acting as enzymes, structural components, and signaling molecules. Proteomics is the large-scale study of the entire set of proteins—the proteome—at a specific time. The proteome is incredibly complex and dynamic, far more so than the genome. A single gene can produce multiple protein variants through alternative splicing, and proteins undergo extensive post-translational modifications (PTMs) like phosphorylation and glycosylation, which alter their function, location, or stability. Furthermore, protein concentrations span a vast dynamic range, making measurement challenging. Key tools include Mass Spectrometry (MS) for identifying and quantifying thousands of proteins simultaneously. Understanding the proteome gives crucial insights into the functional output of our cells, which is vital for finding disease biomarkers and drug targets.
Metabolomics: The Chemical Fingerprint
Metabolomics provides a real-time snapshot of the chemical activity within an organism by studying the metabolome—the complete set of small molecules, or metabolites, such as sugars, lipids, and amino acids. These molecules are the inputs, intermediates, and end products of cellular processes. The metabolome is considered the most direct reflection of an organism’s physiological state (phenotype) because it is influenced not only by genetics but also by diet, lifestyle, medications, and the environment (including the gut microbiome). By measuring metabolites with technologies like Nuclear Magnetic Resonance (NMR) spectroscopy and Liquid Chromatography-Mass Spectrometry (LC-MS), we can map metabolic pathways and find biomarkers that signal disease or track treatment response, offering a powerful readout of cellular health and activity.
The Engine Room: Key Technologies and Data Management
The engine room of ‘omics research consists of sophisticated technologies capable of generating biological data at a massive scale and the powerful computational infrastructure required to analyze it. These high-throughput tools and the bioinformatic methods to process their output are essential to fulfilling the promise of ‘omics, such as finding new ways to fight disease.
Essential Technologies for Data Generation
Breakthroughs in lab technology enable the massive scale of ‘omics research. Key tools include:
- Next-Generation Sequencing (NGS): This technology has revolutionized genomics and transcriptomics. Instead of reading one DNA strand at a time, NGS platforms perform massively parallel sequencing, generating billions of short sequence “reads” at once. This allows scientists to rapidly and affordably sequence entire genomes (DNA) or transcriptomes (RNA), making large-scale population studies feasible.
- Mass Spectrometry (MS): The workhorse of proteomics and metabolomics, MS precisely measures the mass-to-charge ratio of ionized molecules. In a typical workflow, a complex mixture of proteins or metabolites is separated (often by liquid chromatography, or LC) and then ionized. The resulting ions are measured by the mass spectrometer, which generates a spectrum that acts as a molecular fingerprint for identifying and quantifying thousands of different molecules.
- Microarrays: While largely succeeded by NGS for findy, DNA microarrays are still valuable for genotyping, where they efficiently detect the presence of known genetic variations (SNPs) across thousands of samples.
- Emerging Technologies: The field is constantly evolving. Single-cell omics allows researchers to apply genomic, transcriptomic, and other omics techniques to individual cells, revealing cellular heterogeneity that is missed in bulk tissue analysis. Spatial omics technologies go a step further, mapping molecular data back to its original location within a tissue, preserving crucial spatial context.
These high-throughput methods, often aided by laboratory automation, generate the vast datasets required for systems-level biology.
The Data Deluge: Challenges in ‘Omics’ Management
‘Omics technologies generate a deluge of data, creating a significant “Big Data” challenge. A single human genome sequenced at high quality can be over 100 gigabytes, and large-scale projects routinely generate petabytes of data. This requires immense effort to store, organize, and interpret.
- Data Storage and Infrastructure: The sheer size of ‘omics datasets requires robust, scalable, and often cloud-based storage solutions. Public databases like The Cancer Genome Atlas (TCGA) and dbGaP are vital repositories, but managing data access, transfer, and security remains a major logistical hurdle.
- Data Complexity and Heterogeneity: ‘Omics data is multi-dimensional and inherently noisy. A significant challenge is correcting for “batch effects”—systematic variations that arise when samples are processed in different batches or on different days (e.g., due to minor changes in lab reagents or machine calibration). Integrating different data types (e.g., genomic and proteomic) adds further complexity, as each has unique formats and error profiles.
- Statistical and Computational Challenges: Analyzing omics data requires expert bioinformatics skills. A common issue is the “curse of dimensionality” (or p >> n problem), where the number of features measured (p, e.g., 20,000 genes) is far greater than the number of samples (n, e.g., 100 patients). This increases the risk of finding spurious correlations and “overfitting,” where a model performs well on training data but fails on new data. Rigorous statistical methods, quality control, and independent validation are essential.
- Reproducibility and Standardization: For scientific findings to be credible, they must be verifiable by other labs. This requires transparency in data collection methods and analysis pipelines. The adoption of FAIR Data Principles (Findable, Accessible, Interoperable, and Reusable) and standardized workflow management systems like Nextflow and Snakemake are critical for ensuring analyses are reproducible, scalable, and shareable.
Overcoming these challenges with smart computational strategies and strong data governance, as suggested by the Merriam-Webster definition of integrated, is essential for translating ‘omics findings into real-world applications.
From Data to Findy: Applications and Future Horizons
The knowledge gained from ‘omics research is actively solving major challenges in medicine, health, and environmental science. By providing a detailed, multi-layered view of biology, this powerful approach is changing our world in tangible ways.
Changing Medicine and Health
‘Omics is at the heart of the shift towards precision medicine, where treatments are custom to the individual rather than a one-size-fits-all model.
- Personalized Medicine and Pharmacogenomics: By analyzing a patient’s unique molecular profile, doctors can predict disease risk and select the most effective treatments while minimizing side effects. A classic example is pharmacogenomics, which uses genetic data to predict drug responses. For instance, variations in the CYP2C19 gene affect how individuals metabolize the antiplatelet drug clopidogrel. Genetic testing can identify poor metabolizers who require alternative treatments to prevent blood clots.
- Disease Diagnosis and Prognosis: ‘Omics is revolutionizing diagnostics by identifying novel biomarkers. Liquid biopsies, which analyze circulating tumor DNA (ctDNA) from a simple blood draw, use genomics to detect cancer non-invasively, monitor treatment response, and spot recurrence earlier than traditional imaging. The impact of ‘omics on medical diagnosis is providing clearer, earlier insights that save lives.
- Drug Findy and Development: A molecular-level understanding of diseases helps scientists identify and validate new drug targets. For example, by sequencing tumors, researchers can pinpoint the specific mutations driving a cancer and develop drugs that target those vulnerabilities. This approach also helps in understanding mechanisms of drug resistance, enabling the development of next-generation therapies.
- Cancer Research: ‘Omics has revolutionized oncology. The findy that BRAF gene mutations drive over half of all melanomas led to the development of highly effective BRAF inhibitors. Similarly, genomic profiling of tumors is now standard practice in many cancer types to guide the use of targeted therapies and immunotherapies.
- Infectious Disease: In public health, ‘omics is a critical tool for surveillance. During the COVID-19 pandemic, rapid genome sequencing allowed scientists to track the emergence and spread of new variants like Alpha, Delta, and Omicron in near real-time, informing public health policies, vaccine development, and diagnostic testing.
Environmental and Agricultural Science
‘Omics is also making huge strides in protecting our planet and improving our food supply.
- Metagenomics and Microbiome Analysis: This field allows us to study the collective DNA of entire microbial communities directly from their environment (e.g., soil, ocean water, or the human gut). The Human Microbiome Project revealed the vast diversity of microbes living in and on our bodies and linked them to everything from digestion and immunity to mental health. In environmental science, metagenomics helps assess ecosystem health, monitor pollution, and find novel enzymes for industrial applications.
- Crop Improvement and Foodomics: In agriculture, ‘omics helps scientists identify genes for desirable traits like drought resistance, disease tolerance, or higher nutritional value. This accelerates the development of improved crop varieties through techniques like marker-assisted selection. The emerging field of “foodomics” uses these techniques to verify food authenticity, quality, and safety.
- Ecosystem Monitoring and Conservation: Organizations like NOAA use NOAA ‘Omics for environmental research to monitor fisheries, detect harmful algal blooms, and protect endangered species in a faster, more comprehensive way.
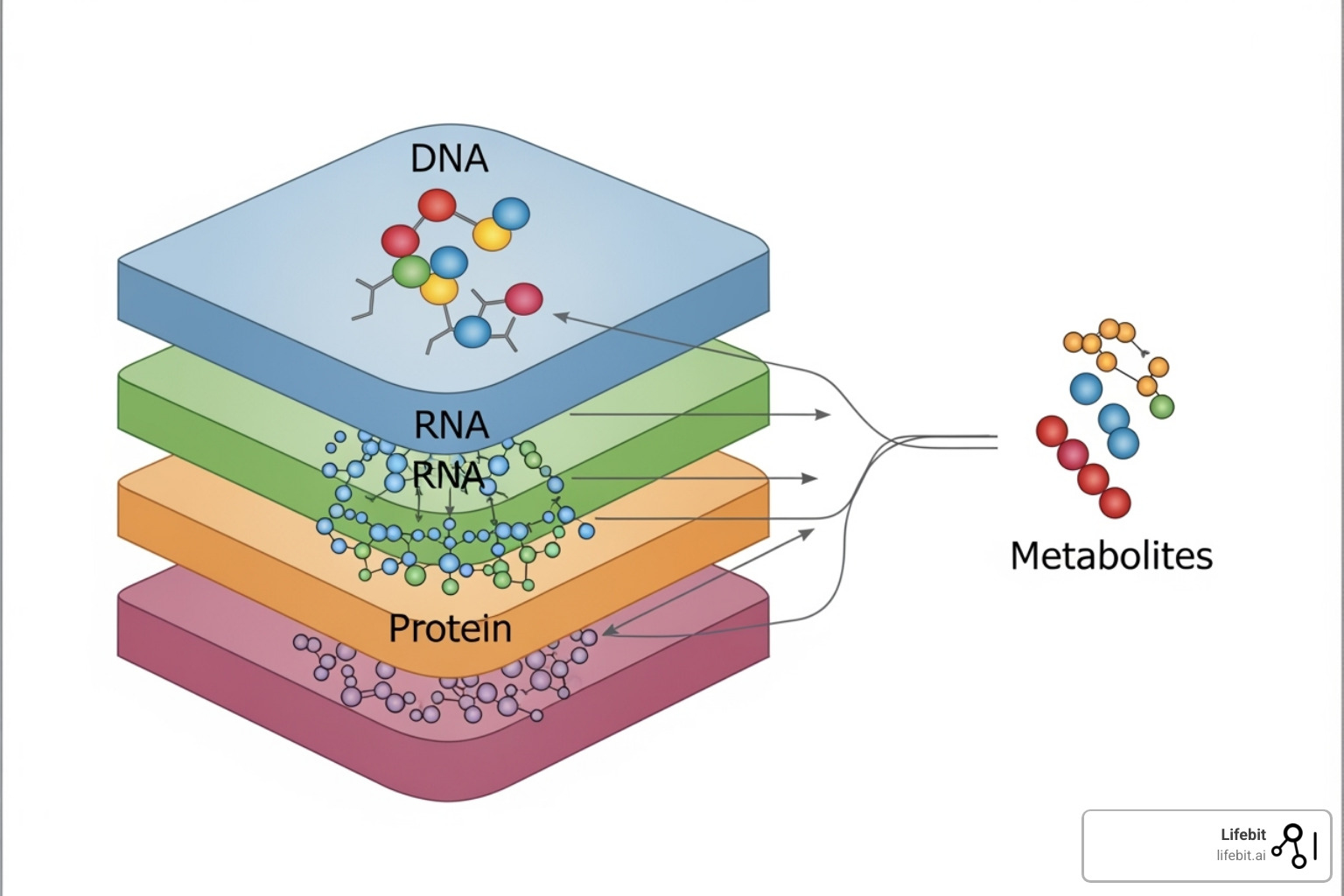
The Future is Integrated: Multi-Omics and Systems Biology
The most exciting frontier is multi-omics: the integration of different ‘omics datasets to create a truly holistic picture. Instead of viewing genes, RNA, proteins, and metabolites in isolation, multi-omics combines them to build comprehensive models of biological systems.
This approach provides a true systems-level understanding, revealing how changes at one level cascade through others. For example, a genomic study might identify a genetic variant associated with a disease. By adding transcriptomic data, researchers can see if that variant alters gene expression. Proteomic data can then show if protein levels or activity are affected, and metabolomic data can reveal the ultimate impact on cellular chemistry. This integrated view is key to solveing the mechanisms of complex diseases.
Another major advance is spatial omics, which adds a critical dimension to molecular analysis: location. Technologies like spatial transcriptomics map gene activity across a tissue slice, revealing how cells interact within their native microenvironment. This is changing our understanding of tumor ecosystems, neurobiology, and developmental processes. While there are significant challenges in data integration, the future of biology lies in combining these diverse data types with powerful computational analysis to decode the complexity of life.
Conclusion
The ‘omics revolution is fundamentally changing our understanding of life. From genomics (the genetic code) and transcriptomics (RNA) to proteomics (proteins) and metabolomics (chemical reactions), ‘omics provides an unprecedented, holistic view of biological systems.
This data-driven approach has already transformed medicine by enabling personalized treatments, accelerating biomarker findy, and improving our fight against cancer and infectious diseases. Beyond human health, ‘omics is a powerful tool for environmental conservation, sustainable agriculture, and understanding the microbial world.
However, the journey continues. The volume, complexity, and heterogeneity of ‘omics data present ongoing challenges in management, analysis, and integration. Ensuring data quality, reproducibility, and secure sharing of insights remains paramount. This is precisely where innovative solutions like the Lifebit platform come into play.
Our next-generation federated AI platform is designed to tackle these challenges, enabling secure, real-time access to global biomedical and multi-omic data. With built-in capabilities for harmonization, advanced AI/ML analytics, and federated governance, Lifebit empowers large-scale, compliant research and pharmacovigilance across biopharma, governments, and public health agencies. Components like our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) deliver real-time insights, AI-driven safety surveillance, and secure collaboration across hybrid data ecosystems.
The ‘omics era represents a paradigm shift from studying isolated parts to understanding integrated systems. As we continue to push the frontiers of biological research, the collaborative power of data, combined with cutting-edge platforms, will undoubtedly open up even more profound findings, ultimately benefiting all of humanity.
To learn more about how we facilitate secure and powerful ‘omics research, we invite you to Explore Lifebit’s federated data solutions.

