Current Systems and Technology in Clinical Trials #1
The Urgent Need for a Clinical Trial Revolution
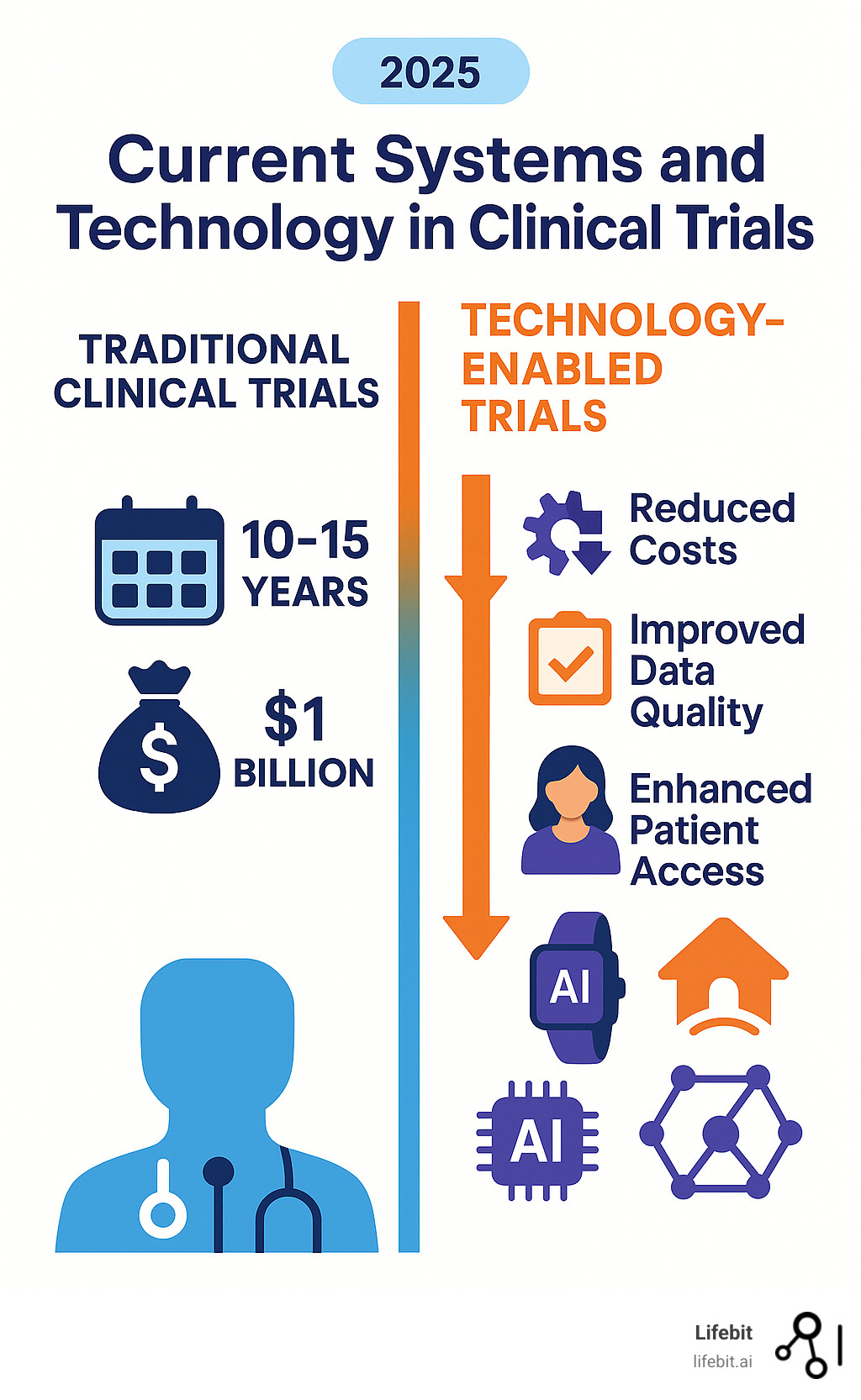
Current systems and technology in clinical trials are changing how we develop medications, but the industry still faces massive challenges. Drug development costs average $1 billion per medication, with timelines stretching over a decade. Only 1 in 7 drugs that enter trials ever reach patients, and fewer than 10% of eligible patients enroll due to geographic and logistical barriers.
These inefficiencies delay critical treatments. Technology offers the solution by making trials faster, more accessible, and patient-centric through innovations like:
- eSource systems for direct, accurate data capture
- Wearables and remote monitoring for continuous real-world data
- Artificial Intelligence (AI) for predictive analytics
- Decentralized Clinical Trials (DCTs) to expand patient access
- EHR integration for real-world evidence
- Federated data networks for secure, privacy-preserving analysis
As Maria Chatzou Dunford, CEO and Co-founder of Lifebit, my experience in computational biology and AI has shown me that the future of clinical research lies in connected, intelligent platforms that put patients first. We can transform global healthcare through federated data analysis in secure, compliant environments.
Related content about current systems and technology in clinical trials:
Core Technological Innovations Revolutionizing Clinical Research
The shift in clinical research is from paper-heavy, error-prone processes to a digital-first, patient-centric approach. This evolution is about reimagining how current systems and technology in clinical trials can work better for everyone by breaking down long-standing barriers. By integrating sophisticated digital tools, we are not just improving efficiency; we are fundamentally enhancing the quality and relevance of the data we collect.

From Manual Entry to eSource: Ensuring Data Integrity
Manual data transcription has historically been a major vulnerability in clinical research, creating error rates of 15-20%. For decisions that impact patient health and determine the fate of billion-dollar therapies, these odds are unacceptable. eSource systems have revolutionized this by capturing data electronically at the point of care, cutting error rates to less than 2%. This includes Electronic Health Record (EHR) data, electronic Patient-Reported Outcomes (ePROs), and electronic Clinical Outcome Assessments (eCOAs).
Beyond accuracy, direct data capture provides real-time access to information for monitors and data managers, allowing for quicker decisions and faster responses to safety concerns. For example, in an oncology trial, an ePRO system allows a patient to report severe side effects from home the moment they occur. This data is instantly available to the clinical team, who can intervene immediately, potentially preventing hospitalization. Studies using eSource platforms see up to 30% faster database lock times and fewer protocol deviations, as data queries can be resolved in near real-time. This makes the entire research process more reliable, compliant, and efficient.
Wearables and Remote Monitoring: Capturing Continuous, Real-World Data
Wearables and remote monitoring devices make it possible to monitor a patient’s health 24/7 outside the controlled environment of a clinic. These sophisticated sensors passively collect high-frequency, detailed health information. A continuous glucose monitor, for instance, can generate thousands of data points daily, providing an unprecedented, granular view into how a treatment impacts a patient’s glycemic control throughout their everyday life.
This continuous data stream gives rise to novel digital biomarkers, which are objective, quantifiable measures of biological and pathogenic processes. For example:
- Neurological Studies: Actigraphy data from a wrist-worn device can track subtle changes in sleep patterns and motor activity in Parkinson’s disease patients, offering a more objective measure of disease progression than periodic clinical assessments.
- Cardiology Trials: ECG patches can detect arrhythmias that might be missed during a short clinic visit, providing critical safety and efficacy data.
- Respiratory Research: Smart inhalers can track medication adherence and usage patterns in asthma patients, correlating it with environmental triggers captured via a smartphone app.
This real-world data offers insights into a patient’s entire day, not just a 30-minute clinic visit. While challenges like data noise and battery life exist, large-scale validation studies consistently show high device accuracy, proving these tools deliver reliable, research-grade data for robust analysis.
You can explore more about how we’re leveraging these insights through digital biomarkers to transform patient care.
Telehealth and eConsent: Bringing Trials to the Patient
Geographic and logistical burdens are primary reasons why many eligible patients cannot participate in trials. Telehealth and eConsent are powerful tools for dismantling these barriers, bringing research directly into patients’ homes.
Virtual consultations, remote monitoring of vital signs, and video-based assessments dramatically reduce patient burden. eConsent has been equally transformative. Instead of dense, multi-page paper forms, patients can engage with interactive, multimedia platforms that use videos, animations, and lay-language summaries to explain the trial. Embedded comprehension quizzes ensure understanding before a digital signature is captured. This process leads to higher comprehension scores, faster enrollment cycles, and increased review time for patients and their families, fostering a more informed and collaborative partnership.
The benefits of telehealth in clinical trials are extensive:
- Increased accessibility by breaking down geographic barriers for rural or immobile patients.
- Reduced patient burden by eliminating travel costs, time off work, and childcare needs.
- Improved patient engagement through more frequent, convenient, and personalized communication.
- Greater flexibility that accommodates real-world schedules, improving adherence.
- Improved safety for vulnerable or immunocompromised populations by reducing exposure risks.
- Faster enrollment and retention by removing the most common logistical obstacles.
These innovations make current systems and technology in clinical trials truly patient-centric, leading to more diverse, representative data and, ultimately, better and more equitable treatments.
The Rise of Decentralized Clinical Trials (DCTs)
Traditional patient recruitment is geographically limited to the radius around a physical trial site, a major factor contributing to an estimated 30% of trial failures due to poor accrual. Decentralized Clinical Trials (DCTs) are a game-changer for current systems and technology in clinical trials by flipping the model: they bring the research to patients, rather than forcing patients to come to the research.
DCTs leverage a suite of digital tools—telehealth, wearables, ePROs, and direct-to-patient logistics—to conduct trial activities remotely. This can range from hybrid trials, which blend in-person visits with remote components, to fully virtual trials conducted entirely in the patient’s home. This paradigm shift enables large-scale remote studies with global recruitment potential, allowing a patient in a rural area to participate in cutting-edge research led by experts thousands of miles away. For a deeper look, A guide to decentralized trials provides comprehensive insights into implementing these transformative methods.
The Operational Shift to Hybrid and Virtual Models
Successfully implementing DCTs requires more than just technology; it demands a fundamental operational shift. Sponsors and CROs must develop new workflows and capabilities, including:
- Direct-to-Patient Logistics: Establishing secure supply chains to ship investigational products, lab kits, and devices directly to participants’ homes.
- Local Healthcare Partnerships: Collaborating with local clinics, mobile nursing services, and diagnostic labs to perform procedures that cannot be done remotely, such as blood draws or infusions.
- New Roles and Training: Creating roles like virtual trial coordinators and providing comprehensive training for both site staff and patients on how to use the digital tools effectively.
- Integrated Technology Platforms: Adopting unified platforms that can seamlessly manage data from various sources (wearables, ePROs, telehealth platforms) to provide a single source of truth for the trial.
Changing Patient Recruitment and Retention
Fewer than 10% of eligible patients enroll in traditional trials, often because the burden of frequent travel and time off work is too high. DCTs dramatically expand access to larger and more diverse patient pools by removing these geographical and logistical limitations. This is particularly transformative for patients with rare diseases, who are often geographically dispersed and may not live near a specialist center.
By reducing participant travel burden, DCTs also lead to significantly improved retention rates. Studies have shown that hybrid DCT models can improve retention by up to 20% compared to traditional site-based trials. Participation fits into people’s lives instead of disrupting them. Furthermore, the use of high passive data capture rates from wearables and remote monitoring devices keeps participants engaged and contributing valuable data without adding to their daily workload.
Enhancing Data Collection and Diversity
While traditional trials offer periodic snapshots of patient health during clinic visits, DCTs provide the full movie. Continuous data streams from wearables, sensors, and apps show how treatments affect people in their real-world context, capturing the variability of daily life. For example, a dermatology trial using patient-uploaded photos can track lesion changes over time, correlating flare-ups with real-world triggers like diet, stress, or environmental factors—insights that are nearly impossible to capture in a sterile clinic setting.
Perhaps most importantly, DCTs achieve broader demographic reach. Traditional trials have historically struggled with diversity, often over-representing populations that live near major academic medical centers. By breaking down access barriers for rural, elderly, minority, and low-income communities, DCTs naturally enroll a more representative patient population. This leads to more diverse, generalizable results and helps us understand how treatments work across different genetic backgrounds, lifestyles, and social determinants of health, making them more effective and equitable for everyone.
The Role of Artificial Intelligence and Machine Learning
Artificial intelligence (AI) and machine learning (ML) have become the computational backbone of modern drug development, changing guesswork into precise, data-driven decisions. Within the ecosystem of current systems and technology in clinical trials, sophisticated AI models are being deployed to process massive genomic, clinical, and real-world datasets. These models can predict outcomes, identify optimal patient populations, design more efficient trials, and even create virtual control groups, all while enhancing patient safety.

AI-Powered Trial Design and Optimization
AI has revolutionized how clinical trials are designed and executed from the very beginning. Key capabilities include:
- Adaptive Trials: AI algorithms and Bayesian statistical models allow trials to modify themselves based on incoming data. This can involve re-allocating patients to more promising treatment arms, adjusting dosages, or stopping a trial early for success or futility, accelerating development phases and improving patient safety.
- Predictive Analytics for Trial Success: ML models can analyze preclinical data, historical trial results, and biomarker information to forecast the probability of a trial’s success. This allows sponsors to invest resources in the most promising candidates and de-risk their development portfolios.
- Optimized Site Selection: Instead of relying on past relationships, AI algorithms analyze historical performance data, patient demographics, and competing trials to identify clinical sites with the highest probability of successful and timely recruitment for a specific protocol.
- Synthetic Control Arms (SCAs): Using carefully selected historical patient data from previous trials or real-world data from EHRs, AI can be used to create virtual (in silico) control groups. This can reduce the number of patients required to receive a placebo, making trials more ethical, faster to enroll, and more appealing to participants.
Intelligent Patient Selection and Stratification
Finding the right patients for a trial is one of the most time-consuming aspects of clinical research. AI has transformed this process, reducing screening time from weeks to mere hours. Machine learning algorithms, particularly those using Natural Language Processing (NLP), can scan millions of unstructured electronic health records—including physicians’ notes, lab reports, and imaging results—to identify ideal candidates who meet complex eligibility criteria almost instantly.
Furthermore, pharmacogenomics leverages AI to analyze patients’ genetic profiles, predicting their likely response to a drug and identifying those at higher risk for adverse reactions with high sensitivity. This allows for patient stratification, ensuring that the right drug is tested on the right patient population, increasing the likelihood of success and improving safety.
AI in Pharmacovigilance and Safety Monitoring
Patient safety is the highest priority in any clinical trial. AI is creating a new paradigm of proactive safety monitoring. Real-time surveillance systems continuously monitor incoming patient data from ePROs, wearables, and lab results for early warning signs of potential adverse events. AI-powered signal detection algorithms can analyze vast, aggregated datasets from multiple sources (including social media and public health databases) to identify potential safety issues with an investigational product much earlier than traditional pharmacovigilance methods. This proactive approach makes current systems and technology in clinical trials not just more efficient, but fundamentally safer for every participant.
Leveraging Integrated Data Ecosystems for Deeper Insights
For decades, clinical research has been hampered by data silos. Valuable patient records, genetic data, imaging files, and sensor readings have been locked away in isolated, incompatible systems, preventing a holistic view of patient health. The power of connected data is now a reality, allowing us to uncover remarkable insights by securely bringing these disparate pieces together. This new era in clinical research is built on the integration of Electronic Health Records (EHRs), the use of Real-World Evidence (RWE), and innovative architectural approaches like federated data networks.
The Role of EHRs and Real-World Evidence (RWE)
EHR integration is the foundation for leveraging clinical data for research purposes. The widespread adoption of FHIR (Fast Healthcare Interoperability Resources) standards has been a game-changer, acting as a universal translator between different healthcare IT systems. FHIR defines a set of common “resources” (e.g., Patient, Observation, Medication) that allow data to be exchanged in a standardized way, breaking down technical barriers to access.
This integration provides access to rich, longitudinal patient data, showing comprehensive health journeys that span years. Pragmatic trials leverage this real-world context to evaluate how treatments perform in routine clinical practice, not just in the idealized conditions of a traditional RCT. They are often lower cost, faster to run, and provide immediately relevant insights for clinicians and payers. Consequently, the use of RWE for regulatory approval has gained significant momentum. For example, Pfizer’s Ibrance (palbociclib) received an expanded FDA indication for male breast cancer based on RWE from EHRs and insurance claims data, demonstrating a pathway for approvals without a conventional trial.
Building Federated Networks and Trusted Research Environments (TREs)
How do you analyze sensitive patient data from multiple hospitals or even multiple countries without violating privacy regulations by moving it? The answer is federated data networks, where the core principle is that the data always stays local.

Instead of centralizing data into a single, vulnerable repository, the analytical code (the algorithm) travels to each institution’s secure data location. The analysis is performed behind the institution’s existing firewall, and only aggregated, anonymized results are returned to the central researcher. Think of it as a librarian who visits different libraries (hospitals) to gather summary statistics about books (patient data) without ever checking the books out or revealing their sensitive contents. This model dramatically simplifies data governance and allows large-scale biobanks and international research programs like the UK’s NHS DigiTrials and Europe’s EHDEN initiative to collaborate without compromising patient privacy. Data security and privacy are paramount, with built-in adherence to regulations like HIPAA and GDPR.
Trusted Research Environments (TREs) provide the secure, controlled digital spaces for this analysis to occur. A TRE is a highly secure computing environment that provides approved researchers with access to sensitive data, but with strict controls that prevent the data itself from being downloaded or removed. This federated approach, executed within TREs, open ups the power of global biomedical data while maintaining the highest possible standards of patient confidentiality and data security.
Navigating the Digital Frontier: Advantages, Challenges, and Governance
Embracing current systems and technology in clinical trials offers unparalleled opportunities to accelerate medical innovation. However, this digital change also presents significant complexities that require thoughtful navigation. We must proactively balance innovation with responsibility to ensure that digital trials are equitable, secure, and scientifically rigorous.

Key Advantages and Persistent Challenges of Current Systems and Technology in Clinical Trials
The shift to digital trials brings a host of remarkable advantages:
- Reduced timelines: Automation, AI-powered recruitment, and real-time data monitoring can cut study phases from years to months.
- Lower costs: Efficiencies in site selection, patient recruitment, and data management significantly reduce administrative and operational overhead.
- Higher data quality: eSource and continuous monitoring provide richer, more accurate, and more objective datasets with fewer errors and missing values.
- Improved patient experiences: Decentralized and hybrid models reduce patient burden, leading to higher engagement, satisfaction, and retention rates.
- Increased diversity and inclusivity: Breaking down geographical and logistical barriers creates more representative research that better reflects the real-world patient population.
However, these benefits are accompanied by persistent challenges that require careful management:
- Implementation costs: The initial investment in new technology platforms, infrastructure, and integration can be substantial.
- Site and patient training: Staff and participants require robust training and ongoing technical support to use new digital tools effectively and confidently.
- Data standardization: Ensuring interoperability and semantic consistency across dozens of different systems, devices, and data sources remains a complex technical task.
- The digital divide: Unequal access to high-speed internet, smartphones, or technology in general risks excluding vulnerable populations (e.g., elderly, low-income, rural), potentially exacerbating health disparities. Solutions include providing pre-configured devices and data plans, offering 24/7 tech support, and designing user interfaces with accessibility in mind.
- Regulatory acceptance: Global regulatory bodies like the FDA and EMA are still developing and refining their frameworks for accepting data from novel digital sources.
- Cybersecurity and scalability: Protecting sensitive patient data from breaches and ensuring that platforms can scale to support global trials with thousands of participants are critical demands.
Ethical, Regulatory, and Data Governance Considerations
As we adopt these advanced technologies, a robust framework for ethical and regulatory oversight is critical. Patient privacy is the central pillar, requiring strict adherence to global regulations like HIPAA in the US and GDPR in Europe. Clear policies on data ownership and the use of modern, interactive informed consent processes are essential to build and maintain patient trust.
We must proactively address cybersecurity risks with end-to-end encryption, access controls, and regular security audits. Regulatory frameworks are evolving to keep pace, and a critical step for acceptance is the validation of digital endpoints. This involves a rigorous process, often following the V3 framework (Verification, Analytical Validation, and Clinical Validation), to prove that a digital measure (e.g., step count from a wearable) is a reliable and meaningful indicator of a clinical outcome. Ensuring data integrity and maintaining clear, immutable audit trails—potentially using emerging technologies like blockchain—are fundamental to upholding the scientific rigor that makes clinical research trustworthy. Federated approaches and trusted research environments are key solutions that help reshape current systems and technology in clinical trials for a more secure, ethical, and effective future.
Frequently Asked Questions about Clinical Trial Technology
As current systems and technology in clinical trials continue to evolve, many people have questions about how these innovations actually work and what they mean for patients and researchers. Let me address the most common concerns I hear from colleagues, patients, and industry professionals.
What is the main goal of using technology in clinical trials?
The primary goal is to make clinical trials faster, more efficient, and more patient-centric. By leveraging technology like AI, wearables, and decentralized platforms, we can reduce timelines by up to 60% and significantly lower those staggering development costs. This gets life-saving treatments to patients sooner by improving data quality, increasing access for diverse populations, and making research a collaborative process that works with them.
How does technology improve patient recruitment?
Technology breaks down the geographical barriers that limit traditional recruitment. Decentralized platforms allow patients to participate from home, opening trials to a global talent pool. AI-powered tools accelerate the process further by scanning millions of electronic health records to identify eligible candidates in hours instead of weeks. This can increase enrollment rates by over 35% and naturally includes more diverse populations, leading to more representative research.
Are digital clinical trials safe and secure?
Yes, safety and security are paramount. Digital trial platforms operate within strict regulatory frameworks like HIPAA and GDPR. Advanced technologies like federated learning and Trusted Research Environments (TREs) improve security by allowing researchers to analyze sensitive data where it resides without ever moving or copying it. The analysis happens locally, and only aggregated insights are shared, never the raw patient data. This design minimizes security risks and protects patient privacy.
The Future is Federated: Strategic Recommendations for Clinical Research
The future of clinical research is accelerating toward hyper-personalized medicine, where treatments are designed for an individual’s unique genetic blueprint and lifestyle. This will be powered by predictive AI models and fully integrated data ecosystems that enable proactive safety monitoring.
Achieving this vision requires a unified, secure platform. The future of current systems and technology in clinical trials is not just about better tools, but a fundamental shift from isolated studies to connected networks and from reactive healthcare to predictive wellness.
Lifebit’s federated AI platform is designed for this future. Our approach ensures sensitive data never leaves its secure location, yet researchers can still collaborate globally. This solves the core challenge of research: accessing necessary data while respecting privacy and security. Our platform components work together to enable this:
- The Trusted Research Environment (TRE) provides secure analysis spaces.
- The Trusted Data Lakehouse (TDL) harmonizes vast, diverse datasets.
- Our R.E.A.L. (Real-time Evidence & Analytics Layer) delivers critical insights.
This enables secure collaboration across hybrid data ecosystems for pharmaceutical companies, government agencies, and research institutions. By combining AI with the security of federated systems, we can accelerate the development of life-changing therapies for everyone. Find how Lifebit’s federated platform is shaping the future of clinical research and see how you can be part of this change.

