Drug Discovery Platforms: Revolutionizing 2025
Revolutionizing Medicine: The Power of Drug Findy Platforms
Drug findy platforms are comprehensive, integrated systems that combine advanced technologies, sophisticated methodologies, and vast datasets to accelerate the identification and development of new medicines. These platforms represent a fundamental paradigm shift, moving the industry away from serendipitous findy and towards engineered, data-driven solutions. They aim to transform healthcare by:
- Significantly speeding up the drug development process, cutting years off traditional timelines.
- Reducing the astronomical costs associated with bringing new drugs to market.
- Improving the overall success rates of new treatments by identifying more viable candidates earlier.
- Expanding the search for novel drug targets, including those previously considered “undruggable.”
The Unyielding Challenge of Traditional Drug Findy
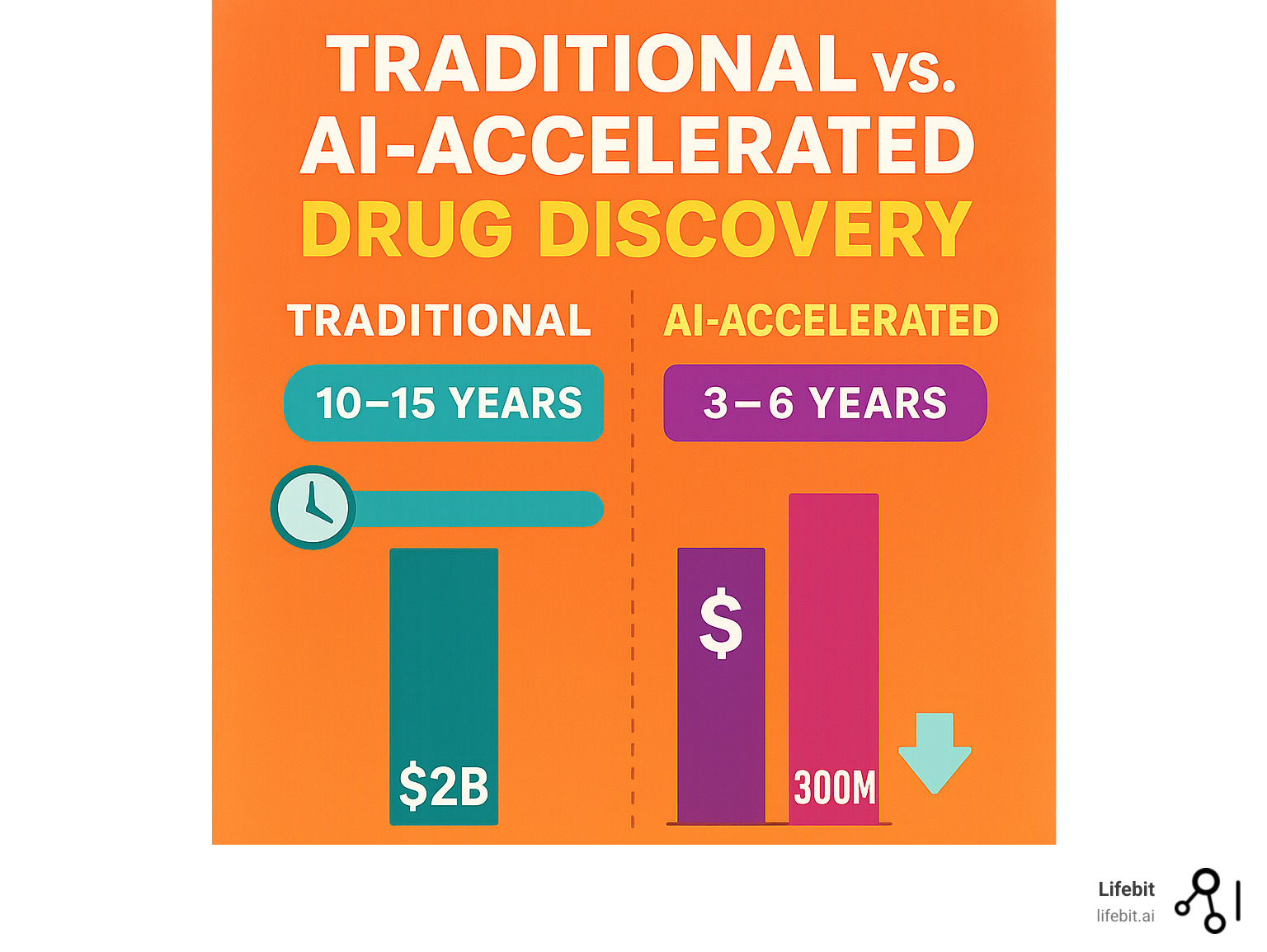
Traditionally, finding new drugs has been an incredibly challenging, inefficient, and costly endeavor. It’s a long, expensive road paved with a staggering rate of failure. The statistics are daunting: the cost to bring a single drug to market often exceeds $2 billion, and the journey from initial research to patient availability can take 10 to 12 years. This phenomenon is sometimes referred to as Eroom’s Law—Moore’s Law spelled backward—highlighting that despite technological advances, the cost of developing a new drug has roughly doubled every nine years since the 1950s.
This inefficiency stems from a multi-stage process fraught with peril:
- Basic Research & Target Identification: This initial phase can take 2-5 years. Scientists must first understand the complex biological mechanisms of a disease to identify a specific target—typically a protein or gene—that a drug could potentially modulate. This process has historically relied on painstaking laboratory work and a degree of serendipity. Many identified targets later prove to be non-causal or unsuitable for drug intervention.
- Lead Findy & Optimization: Once a target is validated, the search for a “lead” compound begins. This involves screening hundreds of thousands, or even millions, of molecules to see if any interact with the target. This High-Throughput Screening (HTS) is resource-intensive and often yields compounds with weak activity or undesirable properties. Promising leads then undergo years of chemical modification (lead optimization) to improve their potency, selectivity, and drug-like characteristics, with no guarantee of success.
- Preclinical Testing: Before any human testing, drug candidates must undergo rigorous preclinical evaluation in cell cultures and animal models to assess their safety and efficacy. This stage can take 1-2 years and is a major attrition point; many candidates are found to be toxic or ineffective in living organisms.
- Clinical Trials: The most expensive and failure-prone stage, lasting 6-7 years on average. Despite immense investment, traditional methods face a massive 90% failure rate during this phase alone.
- Phase I: The drug is given to a small group of healthy volunteers (20-80) to assess its safety, dosage range, and side effects.
- Phase II: The drug is administered to a larger group of patients (100-300) to evaluate its efficacy and further assess its safety.
- Phase III: The drug is tested on a large, diverse patient population (1,000-3,000) to confirm its effectiveness, monitor side effects, and compare it to existing treatments. The vast majority of failures occur in Phase II and III, often after hundreds of millions of dollars have already been spent.
This makes the search for life-saving medicines painstakingly slow and inefficient, leaving many patient needs unmet.
The AI Revolution: Engineering the Future of Medicine
A revolution is underway, powered by Artificial Intelligence (AI) and Machine Learning (ML). These advanced technologies are fundamentally reshaping this landscape, promising to trim timelines, slash costs, and make the drug findy process more predictable and effective. AI algorithms can analyze biological data at a scale and speed impossible for humans, uncovering hidden patterns, identifying novel therapeutic targets, and designing molecules with unprecedented precision. This technological leap is not just an incremental improvement; it’s a transformative force bringing better treatments to patients faster.
AI intervenes at every stage:
- Smarter Target Identification: AI models analyze vast multi-omic datasets (genomics, proteomics, etc.) and scientific literature to identify biological targets that are more likely to be causally linked to a disease, dramatically improving the quality of the starting point.
- Generative Chemistry: Instead of just screening existing molecules, AI can design entirely new ones from scratch (de novo design). These generative models can be optimized to create molecules with high potency, selectivity, and ideal pharmacokinetic properties, significantly increasing the probability of preclinical success.
- Predictive Analytics: ML models can predict a compound’s potential toxicity, how it will be metabolized by the body (ADMET properties), and its likely efficacy before it is ever synthesized in a lab. This in silico (computational) testing weeds out likely failures early, saving immense time and resources.
Democratizing Innovation Through Federated Platforms
Beyond speed and efficiency, AI-enabled platforms are democratizing innovation. Small biotech start-ups, academic spin-outs, and even hospital research groups can now access cloud-based computational power that was once the sole domain of “big pharma.” In the same way that the Human Genome Project ignited a global wave of genomics research, open and federated platforms are lowering the barriers to entry for drug findy, releaseing a new era of collaboration that spans continents and disciplines.
This democratization is built on two pillars:
- Accessible Supercomputing: Cloud providers offer on-demand access to the massive computational resources needed to train complex AI models, turning a prohibitive capital expenditure into a manageable operational cost.
- Collaborative Data Ecosystems: The true power of AI lies in the data it learns from. Large-scale biomedical datasets, such as national biobanks, are invaluable but also highly sensitive and siloed. The solution is not to centralize data, but to bring the analysis to the data.
The Imperative of Security and Federated Governance
Security and governance are paramount. Biomedical data are among the most sensitive forms of personal information, and regulations such as GDPR and HIPAA mandate strict protections. The traditional model of moving data to a central location for analysis creates significant security risks and compliance burdens.
Lifebit meets these requirements through its Trusted Research Environment (TRE) and federated technology layer. This approach allows researchers to “bring the computation to the data” rather than moving data outside secure environments. Here’s how it works:
- Trusted Research Environments (TREs): A TRE is a highly secure, access-controlled digital environment. Approved researchers are given credentials to log in and use a suite of analytical tools on the data, but they cannot download or export the raw data itself. All activity is logged and audited.
- Federated Analysis: When research requires analyzing data across multiple TREs (e.g., in different hospitals or countries), federated technology acts as a secure orchestrator. The analytical query or machine learning model is sent to each location, it runs locally within each secure TRE, and only the aggregated, non-identifiable results are returned to the researcher. This federated approach ensures that privacy is preserved while still giving scientists the analytical firepower they need to make groundbreaking findys.
The impact is already tangible. AI-designed molecules are entering clinical trials in record time, and partnerships between tech companies, biopharma, and public health agencies are generating new insights in areas ranging from rare genetic disorders to antimicrobial resistance. For example, during the COVID-19 pandemic, AI-driven virtual screening helped identify repurposing candidates within weeks— a process that would traditionally have required months of laboratory work and millions of dollars.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. My background in computational biology, AI, and health-tech entrepreneurship has given me a front-row seat to this evolution. It drives our mission at Lifebit to power the world’s leading data-driven drug findy platforms by providing secure, federated access to sensitive biomedical data. When data is the new currency, enabling compliant research across global datasets is the key to open uping the next generation of medicine. This guide will explore how these advanced platforms are reshaping the future of healthcare.
To provide additional context, the World Health Organization estimates that nearly two billion people still lack access to essential medicines—a stark reminder that accelerating findy is not a purely academic exercise but a moral imperative. By converging AI, multi-omic data, and secure cloud technologies, drug findy platforms have the potential to close this gap and deliver therapies to the communities that need them most.
The Building Blocks: A Guide to Drug Findy Platforms
This section will cover the foundational types of drug findy platforms and how they differ. To truly appreciate the revolution happening today, we must first understand the traditional building blocks of medicine and how new technologies are integrating and enhancing them to create the powerful platforms of the future.
Traditional Drug Findy Modalities
For decades, the pharmaceutical industry has relied on three primary modalities for drug development. Each has unique characteristics in terms of size, specificity, and mechanism of action, which dictate its therapeutic applications.
Small Molecule Drugs (SMDs)
Small molecule drugs are the cornerstone of modern pharmacology—the traditional pills and capsules we’re most familiar with. As their name suggests, they are chemically synthesized compounds with a low molecular weight, generally less than 500 Daltons. This tiny size is their greatest advantage, allowing them to easily pass through cell membranes and interact with intracellular targets like enzymes, receptors, and ion channels.
However, their small size and simple structure can sometimes lead to a lack of specificity, causing them to bind to unintended proteins and result in off-target side effects. Think of them as general-purpose keys that might fit several similar locks. Medicinal chemists often rely on frameworks like Lipinski’s Rule of Five to predict whether a small molecule is likely to be orally bioavailable. A major challenge for SMDs has been addressing “undruggable” targets, particularly large, flat protein-protein interaction (PPI) surfaces that lack the well-defined pockets small molecules typically bind to.
Monoclonal Antibodies (mAbs)
Monoclonal antibodies represent a significant leap in complexity and size. These are large, biologically manufactured proteins, typically around 150,000 Daltons. Unlike small molecules, mAbs are designed with exquisite precision to recognize and bind to a single, specific target (an antigen), usually on the surface of cells or circulating in the bloodstream. This high specificity makes them incredibly powerful and often results in fewer side effects. They act like highly specialized keys, designed to fit only one specific lock.
Their mechanisms of action are diverse: they can block signaling pathways (e.g., Herceptin in breast cancer), neutralize circulating inflammatory proteins (e.g., Humira for autoimmune diseases), or flag cancer cells for destruction by the immune system. Because of their large size, they cannot enter cells and must be administered via injection or infusion. Their production in living cell cultures is also a complex and costly process.
RNA-targeted Drugs
This is a newer, rapidly growing class of drugs that includes Antisense Oligonucleotides (ASOs), small interfering RNAs (siRNAs), and messenger RNA (mRNA) therapies. These therapies operate further upstream than SMDs and mAbs by targeting RNA, the messenger molecule that carries genetic instructions from DNA to the protein-making machinery of the cell.
By binding to and degrading, modifying, or in the case of mRNA, supplementing specific RNA molecules, these drugs can prevent or induce the production of proteins at their source. ASO technology, for instance, has already been responsible for first-in-class medicines like Spinraza for spinal muscular atrophy. The global success of mRNA vaccines for COVID-19 showcased the power and speed of this modality. A primary challenge remains delivery; these fragile molecules must be protected by vehicles like Lipid Nanoparticles (LNPs) to reach their target cells intact.
Here’s a quick comparison of these foundational modalities:
| Drug Type | Size (Daltons) | Specificity | Delivery Method |
|---|---|---|---|
| Small Molecule Drugs (SMDs) | < 500 | Lower; can have off-target effects | Oral (pills) |
| Monoclonal Antibodies (mAbs) | ~150,000 | Very High; targets specific extracellular or cell-surface proteins | Injection or Infusion |
| RNA-targeted Drugs (ASOs, siRNA, mRNA) | 5,000 – 1,000,000+ | High; targets specific RNA sequences to modulate protein expression | Injection or Infusion (often with delivery vehicle) |
While the table above illustrates the current therapeutic toolkit, it is important to remember that these categories are beginning to blur. Advances in delivery science, such as lipid nanoparticles and antibody-drug conjugates (which attach a potent small molecule to a targeted antibody), are merging the benefits of small molecules, biologics, and nucleic acid therapeutics into hybrid modalities that promise even greater precision.
The Rise of Computational and AI Drug Findy Platforms
While these modalities form the basis of our therapeutic arsenal, the true revolution lies in how we now find and design them. This is where computational methods and AI come in. An AI Drug Findy Platform leverages a data-driven approach, integrating information about all these modalities. Instead of relying solely on slow and expensive wet-lab experiments, these platforms perform in silico screening, using algorithms to search through vast virtual libraries of compounds to find promising candidates for a specific biological target.
This computational layer doesn’t replace traditional methods but rather augments and accelerates them. It allows researchers to prioritize the most promising molecules for synthesis and testing, dramatically reducing failure rates and costs. The core of this revolution is the integration of multi-omic data to build a holistic understanding of disease biology.
The Power of Multi-Omics
To understand disease, we must look at it from multiple biological layers. AI platforms excel at integrating these “multi-omic” datasets:
- Genomics: The study of the complete set of DNA, including genes. Genomic data can reveal genetic mutations that predispose individuals to certain diseases.
- Transcriptomics: The study of the complete set of RNA transcripts. This shows which genes are active or inactive in a particular cell at a particular time, providing a dynamic view of cellular response.
- Proteomics: The study of the complete set of proteins. Since proteins are the functional workhorses of the cell, proteomics reveals the direct players in biological processes and disease mechanisms.
- Metabolomics: The study of metabolites, the small-molecule end products of cellular processes. This provides a real-time snapshot of a cell’s physiological state.
By integrating these layers, AI can connect a genetic variant (genomics) to a change in gene expression (transcriptomics), which leads to a malfunctioning protein (proteomics) and an altered metabolic state (metabolomics). This systems-biology approach leads to the findy of better targets and more effective drugs across all modalities.
AI Techniques Changing Drug Findy
- AI for Target Identification and Validation: Platforms use Natural Language Processing (NLP) to scan millions of scientific papers and clinical trial records, connecting them with genomic data in massive knowledge graphs. This allows AI to identify novel gene-disease associations and propose targets with strong biological and genetic validation, increasing the likelihood of clinical success.
- AI for Hit Identification and Lead Generation: This is where AI offers a dramatic acceleration. Instead of physically screening millions of compounds (HTS), platforms use Virtual High-Throughput Screening (vHTS). ML models trained on existing bioactivity data can predict the binding affinity of billions of digital compounds in a fraction of the time and cost. Going a step further, De Novo Drug Design uses generative AI models (similar to those that create art or text) to invent entirely new molecules, custom to fit a target’s structure and optimized for properties like potency and low toxicity.
- AI for Lead Optimization and Preclinical Success: A major reason for late-stage failure is poor ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) properties. AI platforms use predictive models to evaluate these properties for a candidate molecule in silico. This allows chemists to iterate and refine a molecule’s structure digitally to perfect its drug-like qualities before committing to expensive and time-consuming synthesis and animal testing.
Real-world Impact
- AlphaFold’s Protein-Folding Revolution: DeepMind’s AlphaFold solved the 50-year grand challenge of predicting a protein’s 3D structure from its amino acid sequence. This breakthrough, now integrated into drug findy platforms, provides accurate structural models for hundreds of millions of proteins. This has opened the door to structure-based drug design for countless targets that were previously intractable due to their unknown structures, saving years of lab work per target.
- Federated Learning for Global Health Crises: During the COVID-19 pandemic, federated analytics, such as those enabled by Lifebit’s technology, allowed international consortia to analyze host genetics from patients across the globe without moving raw data across borders. This maintained compliance and patient privacy while generating rapid, actionable insights into disease severity and identifying drug targets.
- Finding New Antibiotics: Facing a crisis of antimicrobial resistance, researchers are turning to AI. A notable success was the findy of Halicin by an MIT team. They trained a deep learning model to identify molecules with antibacterial properties and finded a powerful new antibiotic candidate with a novel mechanism of action, capable of killing many drug-resistant strains.
- Accelerating Rare Disease Research: AI is uniquely suited to tackle rare diseases, where patient populations are small and data is scarce. By analyzing the underlying genetics and pathways, AI can identify commonalities between different rare diseases, enabling the repurposing of drugs and the design of targeted therapies for previously untreatable conditions.
By weaving together these technologies, drug findy platforms convert the traditional linear “bench-to-bedside” model into an iterative, data-looped engine—one capable of learning from every experiment, every patient, and every data point in real time.

