Why Multi-Omics is Changing Modern Biology
Multi-omics is a research approach that combines data from multiple ‘omics’ layerssuch as genomics, transcriptomics, proteomics, and metabolomicsto create a comprehensive understanding of biological systems.
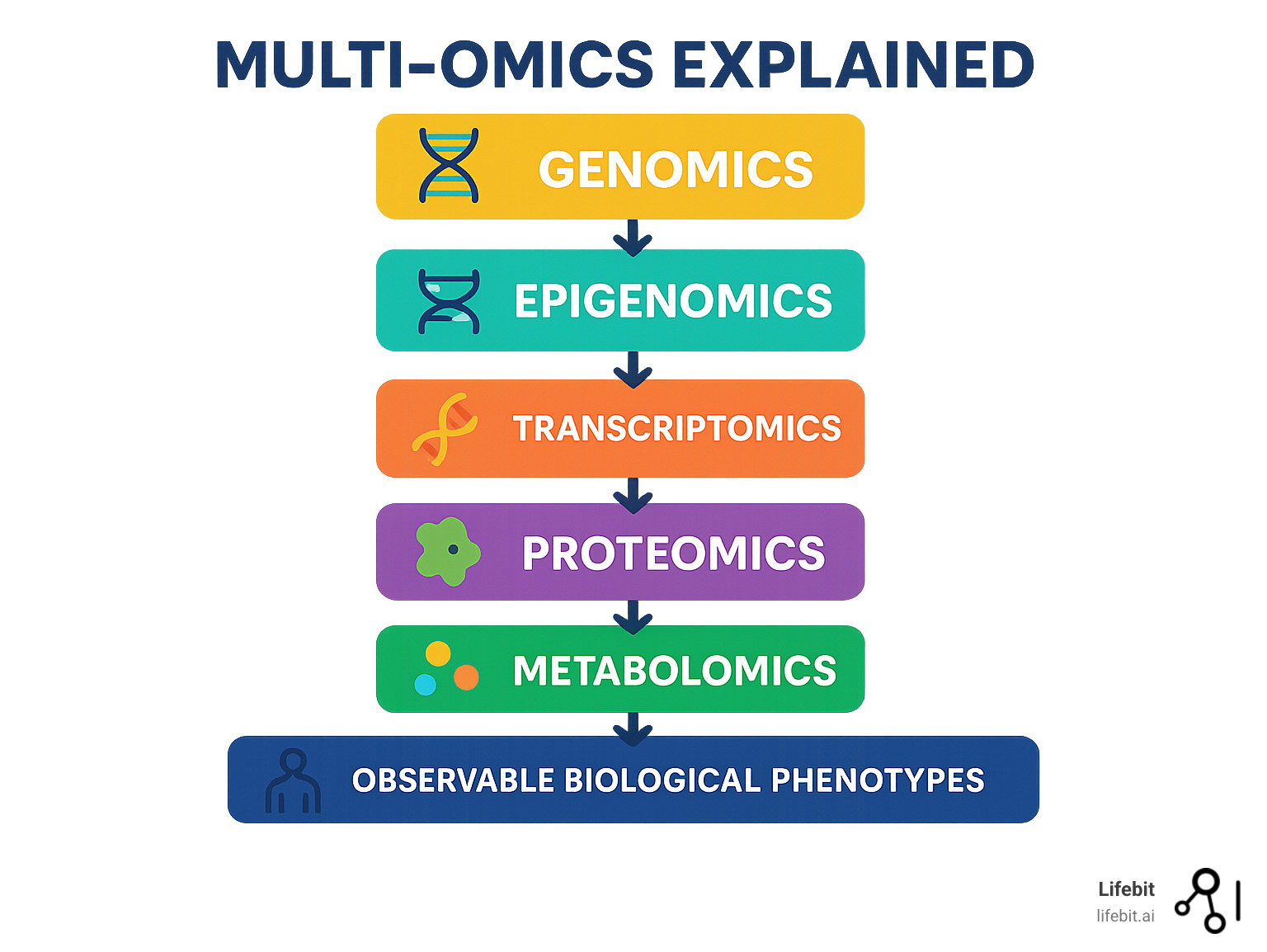
Key Components of Multi-Omics:
- Genomics – Studies DNA structure and genetic variations
- Epigenomics – Examines gene regulation through DNA modifications
- Transcriptomics – Analyzes RNA expression and gene activity
- Proteomics – Investigates protein abundance and function
- Metabolomics – Measures small molecules and metabolic processes
Main Applications:
- Disease research and biomarker finding
- Drug development and personalized medicine
- Understanding complex biological processes
- Predicting treatment responses
Historically, studying individual biological layers provided only small pieces of the puzzle. Multi-omics changes this by integrating multiple data types to reveal the complete flow of information from genes to observable traits.
This integrated approach has already shown remarkable promise. The NIH recently awarded $50.3 million for multi-omics research, while advanced techniques like spatial multi-omics were named among the “seven technologies to watch” by Nature in 2022.
The Symphony of Life: A Deep Dive into the Core ‘Omics’ Technologies
For decades, scientists studied biological systems one layer at a time, like trying to understand a symphony by listening to just one instrument. You might hear the violins (genetics) or the percussion (metabolism), but you miss the harmony, the rhythm, and the emotional depth that only emerge when all parts play together. Life doesn’t work in isolation; it’s a dynamic, interconnected orchestra. Your genes influence your proteins, which catalyze metabolic reactions, which in turn can influence how your genes are expressed. Multi-omics is the conductor’s score, providing a complete toolkit to see how every component is connected and contributes to the final performance—the observable traits of a living organism. To appreciate the power of this integrated approach, first understand the individual instruments.
Genomics: The Foundational Blueprint
Genomics is the study of an organism’s complete set of DNA, known as the genome. This is the fundamental blueprint of life, containing the instructions for building and operating every cell. Genomics focuses on sequencing the DNA code and identifying variations within it. These variations can range from single-nucleotide polymorphisms (SNPs), where a single DNA letter is changed, to larger structural variants like insertions, deletions, and copy number variations (CNVs), where entire sections of genes are duplicated or lost. Technologies like Next-Generation Sequencing (NGS) have made it possible to perform Whole Genome Sequencing (WGS) or Whole Exome Sequencing (WES, which focuses only on the protein-coding regions) at scale. Genomics can identify inherited genetic risk factors for diseases like cancer or cystic fibrosis, but it provides a static picture. It tells us what could happen, but not what is happening in the cell right now.
Epigenomics: The Conductors of the Genome
If genomics is the blueprint, epigenomics is the set of annotations and highlights written on it, dictating which parts are read, when, and how loudly. It studies heritable changes in gene function that do not involve changes to the underlying DNA sequence. These modifications act as a regulatory layer, controlling gene accessibility and expression. Key epigenetic mechanisms include:
- DNA Methylation: The addition of a methyl group to DNA, which typically acts to silence genes.
- Histone Modification: Chemical modifications to histone proteins (around which DNA is wrapped), which can either compact the DNA to restrict access or loosen it to allow for gene expression.
- Chromatin Accessibility: The physical openness of the DNA, determining which genes are available for transcription.
Technologies like Bisulfite Sequencing (for methylation), ChIP-Seq (for histone modifications), and ATAC-Seq (for chromatin accessibility) allow scientists to map these regulatory marks across the genome. Epigenomics provides a crucial link between the environment (e.g., diet, stress) and the genome, explaining how external factors can change gene expression patterns over a lifetime.
Transcriptomics: The Active Musical Score
Transcriptomics moves from potential to action by analyzing the transcriptome—the complete set of RNA transcripts in a cell at a specific moment. RNA, particularly messenger RNA (mRNA), is the transcribed copy of a gene’s instructions, ready to be translated into a protein. By measuring the abundance of every RNA molecule, transcriptomics reveals which genes are actively being expressed and at what levels. It provides a dynamic snapshot of cellular activity. The primary technology used today is RNA-sequencing (RNA-seq), which has largely replaced older microarray technologies. Transcriptomics is incredibly powerful for comparing cellular states—for example, identifying which genes are upregulated in a cancer cell compared to a healthy cell—and for understanding how cells respond to stimuli or treatments.
Proteomics: The Instruments in Action
Proteomics is the large-scale study of proteins, the true workhorses of the cell. If mRNA is the musical score, proteins are the instruments actually playing the music. They perform a vast array of functions: catalyzing reactions (enzymes), providing structure (collagen), transporting molecules (hemoglobin), and sending signals (hormones). Proteomics aims to identify and quantify all the proteins in a cell (the proteome) and also characterize their post-translational modifications (PTMs), which can switch their function on or off. The primary technology is mass spectrometry (LC-MS/MS), which can identify thousands of proteins from a complex sample. Proteomics is challenging due to the immense dynamic range of protein abundance (from millions of copies per cell to just a handful) and the complexity added by PTMs. However, it provides a direct view of the functional machinery of the cell, which is often more closely related to phenotype than transcriptomics, as mRNA levels don’t always perfectly correlate with protein levels.
Metabolomics: The Resulting Acoustics
Metabolomics is the study of the metabolome—the complete set of small-molecule chemicals, or metabolites, found within a biological sample. This includes sugars, lipids, amino acids, and other products of cellular processes. Metabolites are the final downstream output of genomic, epigenomic, and proteomic activity. As such, the metabolome is considered the closest link to the organism’s observable phenotype (e.g., health status, disease state). It provides a real-time chemical fingerprint of cellular physiology. Technologies like Mass Spectrometry (MS) and Nuclear Magnetic Resonance (NMR) spectroscopy are used to measure hundreds to thousands of metabolites simultaneously. By analyzing the metabolome, scientists can identify disrupted metabolic pathways in disease or find biomarkers that reflect the body’s current state.
From Data to Findings: Integrating and Analyzing Multi-Omics Data
The Next Frontier: Single-Cell and Spatial Multi-Omics
Traditional multi-omics analyzes bulk tissue samples, averaging the molecular profiles of millions of cells. This masks the incredible diversity within tissues, much like studying a city using only its overall statistics. Single-cell and spatial multi-omics overcome this limitation by providing a much higher-resolution view.
Single-Cell Multi-Omics: Solving Cellular Diversity
Single-cell multi-omics allows us to isolate and profile thousands of individual cells, one by one. This gives us unprecedented resolution to uncover tissue heterogeneity and link genotype to phenotype at the single-cell level. We can now ask how a genetic variation affects the molecular profile of a specific cell.
Technologies like scRNA-seq (gene expression), scATAC-seq (chromatin accessibility), and CITE-seq (RNA and protein expression) can even measure multiple ‘omic’ layers from the same single cell. These advances are crucial for ambitious projects like the Human Cell Atlas, which aims to map every cell type in the human body. Through these methods, researchers have found previously unrecognized cell types and new cell states associated with diseases like cancer and Alzheimer’s. More details can be found in this resource on single-cell strategies and applications.
Spatial Omics: Adding a Map to the Data
While single-cell analysis provides incredible detail, it typically requires separating cells from their tissue, which means we lose crucial spatial context. A cell’s behavior is heavily influenced by its neighbors and local microenvironment.
Spatial multi-omics solves this by analyzing tissues in situ, mapping the ‘omes’ of cells while keeping them in their original positions. This allows us to study cell-cell interactions and tissue architecture, revealing how cellular neighborhoods contribute to function or disease. Technologies like in-situ sequencing and imaging mass cytometry are developing so rapidly that spatial multi-omics was named one of seven technologies to watch in a 2022 Nature article. By combining single-cell resolution with spatial context, we are gaining an unprecedented ability to decode biology with extraordinary precision.
Changing Medicine: Key Applications and Future Directions
The power of multi-omics extends far beyond the laboratory, changing how we understand, diagnose, and treat disease. By weaving together insights from multiple biological layers, this approach is bringing us closer to truly personalized medicine.

Applications in Health and Disease
The impact of multi-omics is felt across nearly every area of medical research. A key goal in all applications is biomarker identification—finding molecular signatures that can predict disease or guide therapy.
- Cancer research: Multi-omics reveals the full picture of how genetic mutations, epigenetic changes, and metabolic shifts drive tumors. This helps classify tumors more precisely and identify new treatment targets, as shown in recent research advancing cancer research with multiomics.
- Infectious diseases: This approach helps us understand the complex interplay between pathogens and the host immune system. During the COVID-19 pandemic, researchers used it to understand why disease severity varied so widely among patients.
- Neurodegenerative diseases: For conditions like Alzheimer’s, multi-omics integrates genetic risk with changes in brain proteins and metabolites to identify early warning signs and potential intervention points.
- Immunology: The field of systems vaccinology uses multi-omics to design more effective vaccines by mapping the complete immune response to vaccination.
- Cardiovascular and chronic diseases: By combining genetic risk factors with profiles of blood lipids, inflammatory markers, and metabolites, researchers can identify high-risk individuals for heart disease, diabetes, or kidney disease long before symptoms appear.
Major Initiatives and the Future of Multi-Omics
The transformative potential of multi-omics has sparked massive collaborative efforts. The Human Microbiome Project integrated patient data with multiple ‘omic’ datasets to understand how our microbial partners influence health. More recently, The NIH Multi-Omics for Health and Disease (MOHD) program allocated $50.3 million to advance these applications in diverse populations, with a focus on integrating environmental and social factors. You can learn more about this initiative at The NIH Multi-Omics for Health and Disease (MoHD) program.
The ultimate goal is personalized medicine, moving away from a “one-size-fits-all” approach. By understanding each person’s unique molecular profile, doctors can tailor treatments with unprecedented precision. Even more exciting is the promise of predictive health—using molecular profiles to identify disease risk years before symptoms appear, shifting healthcare from reactive to proactive.
Frequently Asked Questions about Multi-Omics
As multi-omics continues to transform biology, many common questions arise about this powerful approach.
What is the difference between genomics and multi-omics?
Genomics is like having the complete blueprint for a house—it reveals the foundational structure and potential. Multi-omics is like having that blueprint plus a real-time view of the house in action: which rooms are used (transcriptomics), what activities are happening (proteomics), and what supplies are consumed (metabolomics).
In short, genomics provides the static genetic code, while multi-omics captures the dynamic, living story of how that code is expressed and regulated. It connects the genetic cause to the biological effect.
How is multi-omics used in personalized medicine?
Multi-omics provides a detailed picture of each individual’s unique biological signature, which is the foundation of personalized medicine. It allows clinicians to:
- Stratify patients into precise subgroups based on their molecular profiles, not just their symptoms.
- Predict drug responses to select the most effective treatment and avoid adverse effects.
- Identify novel drug targets by revealing disrupted biological pathways.
- Enable proactive healthcare by detecting disease risk long before symptoms appear, allowing for early intervention.
Is multi-omics only for human biology?
Not at all. While human health is a major focus, multi-omics is a versatile approach used across many fields of biology.
In agriculture and plant science, it’s used to develop more resilient and nutritious crops. In environmental science, it helps researchers understand how ecosystems respond to climate change. Microbiology uses it to study how bacterial communities function and how pathogens evolve. Any complex biological system—from a single cell to an entire ecosystem—can be studied using a multi-omics approach.
Conclusion
We’ve journeyed through multi-omics, seeing how integrating genomics, proteomics, and other layers provides a holistic view of life. This represents a fundamental paradigm shift in biology—moving from studying isolated parts to understanding complex, interconnected systems.
The power of data integration, amplified by AI and machine learning, is open uping insights that were previously impossible. We are uncovering the hidden patterns that govern health and disease.
The future potential is breathtaking. We are moving toward a world of truly personalized medicine, where treatments are custom to each person’s unique molecular signature and diseases can be predicted and prevented before they start.
At Lifebit, we are passionate about turning this potential into reality. Open uping the full promise of multi-omics requires robust, secure, and scalable technology. Our next-generation federated AI platform enables researchers to securely access and analyze global biomedical data, accelerating groundbreaking findings.
We power large-scale, compliant research for biopharma, governments, and public health agencies, helping them transform data into life-saving insights. To learn how we can help your organization, visit our solutions page.

