An Essential Guide to Disease Registers and How to Find Information
Why Disease Registers Are Essential for Modern Healthcare
Disease registers are organized systems that collect and analyze health data about specific conditions across populations. They are powerful tools for tracking disease trends, supporting clinical research, improving patient outcomes, monitoring treatment safety, informing public health policy, and enabling pharmacovigilance for drug safety.
These registers have become critical infrastructure in healthcare, especially as chronic diseases affect over 130 million Americans. They provide the longitudinal data needed to understand disease progression, treatment efficacy, and complication prevention.
However, managing health data across disparate systems, ensuring patient privacy, and maintaining data quality presents significant challenges. Healthcare organizations, regulatory bodies, and pharmaceutical companies need scalable solutions to access diverse datasets while maintaining compliance and security.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in computational biology and biomedical data platforms, my background in genomics, AI, and health-tech has given me deep insights into how federated data analysis can transform how we collect and analyze registry data globally.
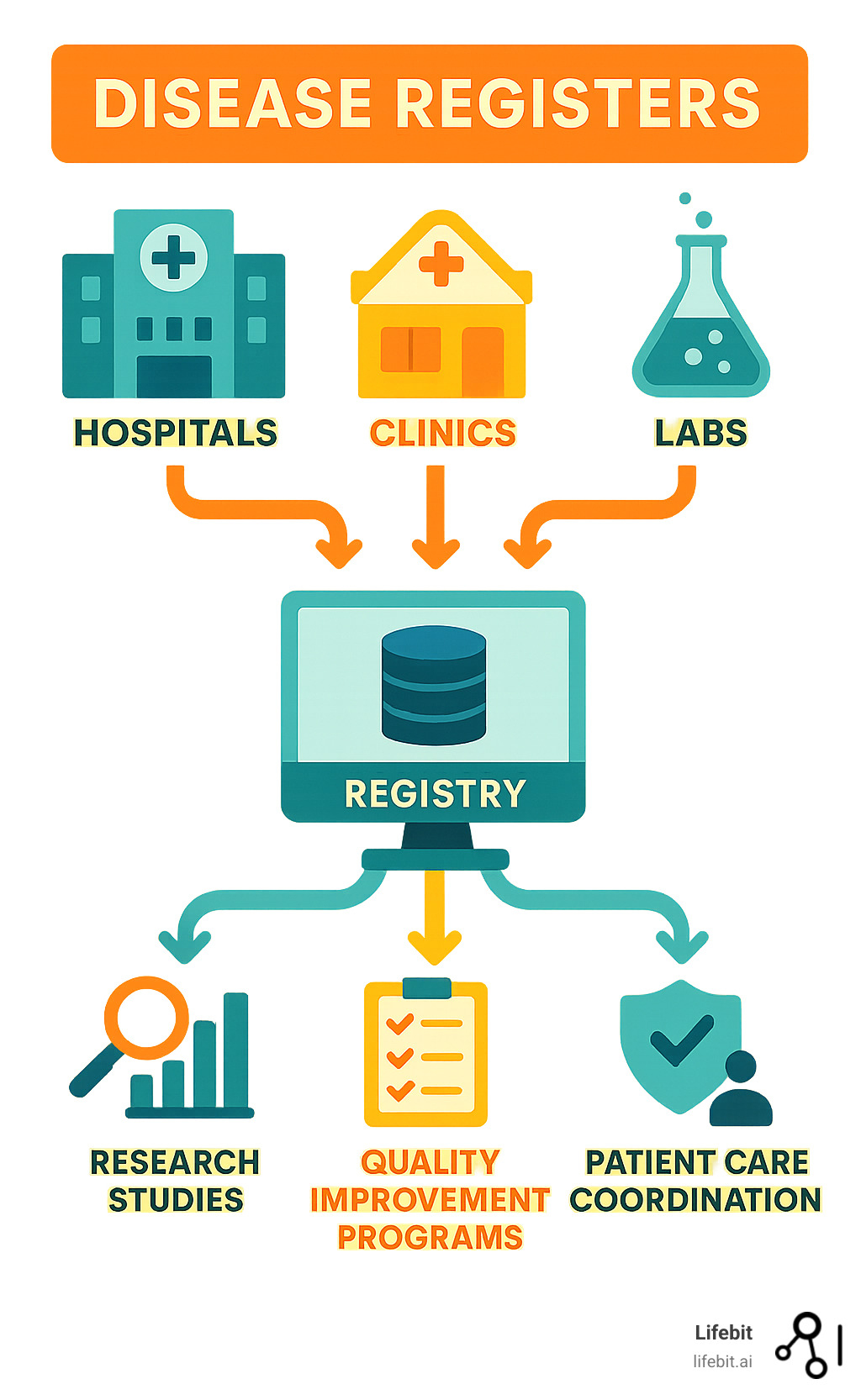
What Are Disease Registers and Why Are They Crucial?
A disease registry is a specialized system that collects, stores, and analyzes health information about people with the same medical condition. More than a simple patient list, it’s a dynamic database that gathers meaningful data to understand how diseases affect people in the real world.
Registries are crucial for population health management, tracking disease progression, treatment efficacy, and risk factors. The National Committee on Vital and Health Statistics has long recognized their importance for understanding the true impact of health conditions across communities.
With chronic diseases accounting for 75% of all medical care costs in the United States, the need for reliable data is clear. Disease registers provide the organized, long-term information required to tackle these challenges.
The Core Goals of a Disease Registry
Every disease registry is built with specific goals, but they share common purposes that make them valuable. At their core, registries aim to improve patient outcomes by helping doctors make informed care decisions based on real-world treatment data. They also monitor disease trends across populations, support clinical research by capturing how treatments perform in everyday settings, and assess healthcare quality by allowing providers to benchmark their performance.
Furthermore, registries play a vital role in evaluating treatment safety through post-market surveillance and informing public health policy with evidence-based insights for resource allocation and prevention strategies.
How Registries Differ from EMRs and Clinical Trials
It’s important to distinguish disease registers from other health data systems.
| Feature | Disease Registries | Electronic Medical Records (EMRs) | Clinical Trials |
|---|---|---|---|
| Purpose | Collect specific data for a defined condition across populations to understand disease patterns and improve care | Document individual patient care for clinical management and billing purposes | Test specific new treatments under highly controlled conditions |
| Population Scope | Defined groups of patients with specific diseases, often from multiple healthcare facilities | Individual patient records within a single healthcare system | Carefully selected patients meeting strict research criteria |
| Data Type | Focused information relevant to the specific disease being studied | Comprehensive clinical data covering all aspects of patient care | Highly structured data points directly related to the treatment being tested |
| Study Design | Real-world observation of how diseases and treatments behave in everyday settings | Record-keeping system for clinical care | Experimental studies designed to prove whether treatments work |
In short, EMRs are for individual patient care, and clinical trials test new interventions in controlled settings. Disease registers bridge this gap by observing how diseases and treatments behave in the real world, providing a complete picture of how conditions affect diverse communities over time.
The Benefits of Disease Registers Across Healthcare
Disease registers are more than just databases; they are active systems that connect patients, doctors, researchers, and public health officials to drive meaningful change. By changing data into action, registries create opportunities for better treatments, safer medications, and smarter healthcare decisions, proving their value in today’s Pay-for-Performance (P4P) programs and value-based care models.

Benefits for Patients and Healthcare Providers
For patients, participating in a registry can lead to access to new treatments and clinical trials. It also offers a chance to contribute to science, knowing their health journey could improve future outcomes. For providers, simple patient registries can dramatically improve chronic disease care. They enable performance benchmarking against national averages and help identify at-risk patients through care reminders and proactive alerts, which can prevent complications and save lives.
Impact on Research and Public Health
Registries provide the real-world evidence (RWE) that bridges the gap between controlled clinical trials and everyday healthcare. Their longitudinal data reveals long-term patterns, which is especially crucial for rare diseases where gathering sufficient information is a challenge. For public health, registries are essential for pharmacovigilance, acting as an early warning system for medication safety. Agencies like the Agency for Toxic Substances & Disease Registry (ATSDR) use them to track disease prevalence, identify risk factors, and inform health service planning.
Driving Quality Improvement and Cost-Effectiveness
Registries are revolutionizing healthcare value and quality. They promote adherence to clinical guidelines and help reduce care variation, ensuring patients receive high-quality, standardized treatment. In the shift toward value-based care, registries provide the evidence to identify effective treatments by comparing real-world outcomes. By optimizing preventive care, registries can prevent diseases before they start, which is more cost-effective than treating advanced conditions. As demonstrated in Phase IV drug surveillance, patient-centered registries are invaluable for ensuring medications remain safe and effective in real-world settings.
Types, Data, and Technical Aspects of Disease Registers
Behind every successful disease registry is a sophisticated technical architecture designed to be reliable, secure, and powerful enough to handle massive amounts of sensitive health data. This backbone ensures data integrity, protects patient privacy, and enables the groundbreaking medical insights that can save lives.
Common Types of Disease Registers
Registries are designed for different purposes:
- Health Services Registries track specific medical procedures, such as joint replacements or cardiac surgeries, to monitor long-term performance and outcomes.
- Disease and Condition Registries focus on specific illnesses. Cancer registries are a prime example, tracking diagnosis, treatment, and survival, while diabetes registries help manage chronic care.
- Product Registries monitor medical products post-market. This includes medical device registries for items like pacemakers and pharmaceutical registries for new drugs.
- Pregnancy Registries, like the Pregnancy & Cancer Registry, monitor health outcomes when pregnancy intersects with other medical conditions.
Data Collection and Management
Registries collect detailed, standardized information to tell a complete patient story. Key data elements include demographics, diagnosis codes (e.g., ICD-10), clinical measurements (lab results, scans), treatment information, and Patient-Reported Outcomes (PROs) that capture the patient’s perspective.
Data is sourced from electronic medical records, labs, insurance claims, and patient surveys. The primary technical challenges are data standardization and interoperability—ensuring information from different sources can be combined meaningfully. The AHRQ guide on computerized disease registries offers solutions to these challenges.
Ensuring Patient Privacy and Data Security
Protecting sensitive health data is fundamental. The process begins with transparent patient consent. Data de-identification techniques like pseudonymization and aggregation are used to remove personally identifiable information, protecting patient privacy.
Strict adherence to regulations like HIPAA compliance in the US and GDPR in Europe is mandatory. Secure data access is controlled through robust authentication systems, ensuring only authorized personnel can view specific data.
Federated data models improve security by bringing the analysis to the data’s location, rather than moving sensitive data. This approach dramatically improves privacy and simplifies compliance, enabling large-scale research across institutions while keeping patient information secure within its original environment. Ethical considerations regarding data ownership and transparency are also paramount.
How to Find and Participate in a Disease Registry
Whether you are a patient, researcher, or healthcare provider, finding a relevant disease registry is more accessible than ever. With the right resources, you can connect with registries that align with your specific condition, research interests, or clinical focus.

Where to Search for Disease Registers
Start your search with trusted, authoritative sources:
- Government portals like the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC) in the US, or the National Disease Registration Service (NDRS) in the UK, list registries that meet high standards.
- Patient advocacy groups are deeply connected to their communities and often have information on relevant research initiatives.
- Clinical trial databases such as ClinicalTrials.gov often list studies that are connected to or feed data into long-term registries.
- Academic institutions, research centers, and professional medical societies frequently host specialized registries.
When searching, look for registries that are transparent about their goals, data use, and privacy protections.
Examples of Prominent Registries
To illustrate the variety available, here are some well-established disease registers:
- Cancer Registries: The SEER registries (Surveillance, Epidemiology, and End Results Program) are the gold standard for cancer data collection in the US, shaping prevention and treatment strategies.
- Rare Disease Registries: The National ALS Registry and the Cystic Fibrosis Foundation Patient Registry have been instrumental in advancing the understanding and care of these conditions.
- Chronic Condition Registries: The RESONANCE Registry for pericarditis collects real-world data to help doctors understand treatment efficacy for this specific heart condition.
- Device Registries: The American Joint Replacement Registry monitors hip and knee replacement outcomes, helping improve implant choices and surgical techniques.
Challenges and the Future of Disease Registries
While disease registers are incredibly valuable, traditional models face significant challenges that can limit their effectiveness. However, a technological revolution is underway that is changing how registries work, moving from simple digital databases to intelligent, connected systems.
Overcoming Common Problems
Traditional registries often struggle with several key issues:
- Data quality: Inaccuracies and incomplete information from manual data entry can compromise analyses.
- Funding and sustainability: High costs for building and maintaining registries can threaten their long-term viability.
- Interoperability: A significant number of registries (51%) are not linked to clinical data from electronic health records, trapping valuable insights in silos.
- Clinician and patient burden: Time-consuming data entry can lead to incomplete data and registry abandonment.
The Next Generation: AI and Federated Learning
The future of disease registers is being shaped by artificial intelligence and federated learning.
Artificial Intelligence (AI) and Machine Learning (ML) are revolutionizing data handling. AI can automate data extraction from clinical notes, use predictive analytics to identify at-risk patients, and deliver real-time insights, reducing manual work and accelerating research.
Federated technology solves the critical challenge of analyzing data from multiple institutions without compromising patient privacy. Instead of moving sensitive data, the analysis algorithms travel to where the data resides. This “analyzing data without moving it” approach offers improved privacy and massive scalability for Real-World Evidence (RWE) by combining insights from global sources without sharing raw data. The result is truly patient-centric research, where individuals can contribute to medical knowledge while their personal information remains protected within their local healthcare system.
Conclusion
Disease registers have evolved from simple lists into sophisticated, vital tools for modern healthcare research and quality improvement. They empower patients, guide clinicians, and provide researchers with the real-world evidence that clinical trials cannot. Given that chronic diseases drive 75% of US medical care costs, the data from these systems is essential for public health planning and prevention.
The future of disease registers is even more promising. Challenges like data quality and interoperability are being overcome by innovative technologies. Artificial intelligence and machine learning are automating data processing and delivering predictive, real-time insights.
Most importantly, federated technology is enabling analysis of data where it lives, without moving sensitive patient information. This creates opportunities for massive, global research collaborations while ensuring patient privacy remains intact. This shift toward connected, secure data ecosystems represents a fundamental change in biomedical research, allowing access to insights from globally distributed datasets in real-time.
At Lifebit, we are pioneering this change. Our federated AI platform enables secure, real-time access to global biomedical data for research and pharmacovigilance. By fostering secure collaboration, we are making disease registers more powerful tools for improving health outcomes worldwide.
Learn how Lifebit’s federated platform powers secure research on sensitive data and find how this technology is changing the landscape of disease registers and biomedical research.

