Genomics Unraveled: What Your DNA Can Tell You
Genomics: Unravel DNA – The #1 Guide
Solving the Blueprint: What is Genomics?
Genomics is the study of an organism’s complete set of DNA, known as its genome. This exciting field explores all genes, their functions, and how they interact with each other and the environment. It’s all about understanding the entire genetic blueprint of life, from the simplest bacterium to the most complex human being.
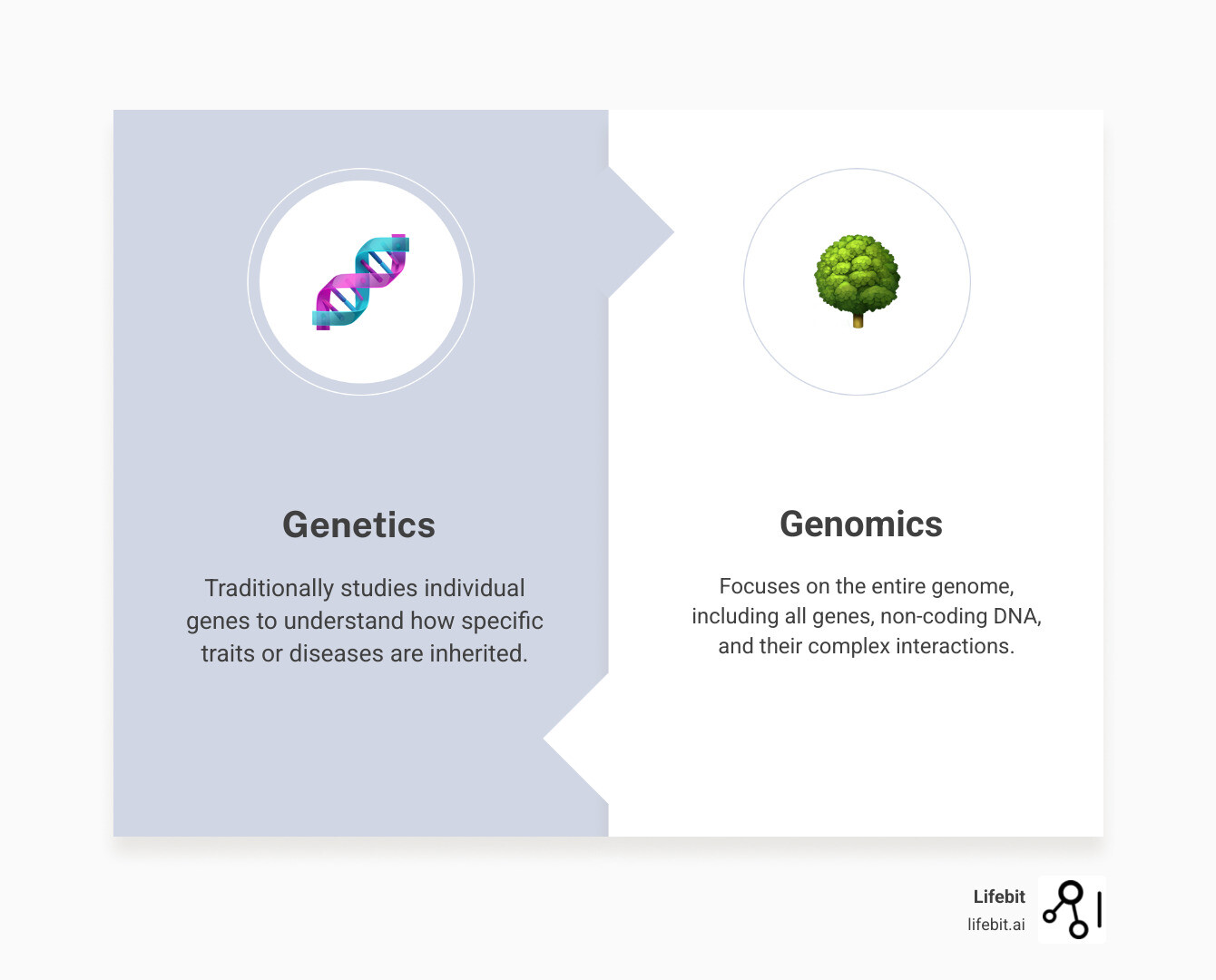
Here’s a quick look at how genomics differs from traditional genetics:
- Genomics: Focuses on the entire genome. This includes all genes, non-coding DNA, and the complex interactions between them. It’s about seeing the whole forest, not just individual trees, to understand the system as a whole.
- Genetics: Traditionally studies individual genes. It looks at how specific traits or diseases are passed from one generation to the next, often focusing on a single gene’s role.
Genomics uses powerful tools to map, sequence, and analyze genomes. It helps us understand how our bodies work, why we get sick, and how we can find new treatments. This broader view has sparked an “omics” revolution, giving rise to related fields like transcriptomics (studying RNA) and proteomics (studying proteins), all aimed at capturing a complete biological picture. This systems-level approach is changing how we approach biology and medicine.
As Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit, I have dedicated over 15 years to computational biology, AI, and high-performance computing, changing healthcare through federated data analysis in genomics. My work has focused on building cutting-edge tools to empower precision medicine and biomedical data integration.
The Blueprint of Life: From Genetics to Genomics
Imagine a world before we truly understood the instruction manual for life. For centuries, scientists puzzled over heredity, trying to grasp how traits passed from parent to child. This journey began with simple observations and evolved into the complex, interconnected field of genomics we know today.
Our story starts in a monastery garden with a curious monk named Gregor Mendel. His meticulous pea plant experiments in the mid-19th century laid the groundwork for genetics, revealing that traits are inherited in predictable patterns. Fast forward to the mid-20th century, and the scientific world was abuzz with the race to uncover the structure of DNA. In 1953, James Watson and Francis Crick, building on crucial X-ray diffraction work by Rosalind Franklin and Maurice Wilkins, unveiled the iconic double helix, forever changing our understanding of life’s fundamental molecule.
The ability to “read” this molecule was the next great leap. Fred Sanger’s pioneering methods in the 1970s made DNA sequencing a reality, first for proteins like insulin, then nucleic acids. In fact, the first nucleic acid sequence ever determined was the ribonucleotide sequence of alanine transfer RNA in 1964. His team later sequenced the entire genome of bacteriophage MS2-RNA, a tiny virus with just four genes in 3569 base pairs, in 1976. This was followed by the first fully sequenced DNA-based genome, bacteriophage φX174, with its 5,386 nucleotides, in 1977. These early efforts, though painstaking, proved that reading an entire genome was possible.
The World Health Organization provides clear definitions for these terms: WHO definitions of genetics and genomics.
Foundational Findings
The journey to modern genomics is paved with groundbreaking findings that progressively revealed the intricate workings of life. Beyond the DNA structure itself, the chromosome theory of inheritance proposed that genes are located on chromosomes, which carry hereditary information. This was a crucial conceptual step, moving us from abstract units of inheritance to physical structures within the cell.
Then came the central dogma of molecular biology, neatly stating that information flows from DNA to RNA to protein. This fundamental principle explained how the genetic code translates into the building blocks and machinery of life. The sequencing of the first RNA molecule (alanine tRNA) in 1964 and the first DNA-based genome (bacteriophage φX174) in 1977 were monumental achievements. These early successes, though small in scale by today’s standards, demonstrated the feasibility of reading entire genetic codes.
As technology advanced, so did our ambition. The human mitochondrion genome, a smaller, circular DNA molecule within our cells, was fully sequenced in 1981, revealing 16,568 base pairs (about 16.6 kb). This was a significant step as it represented the first complete eukaryotic organelle genome. In 1992, the first eukaryotic chromosome, chromosome III of brewer’s yeast Saccharomyces cerevisiae (315 kb), was sequenced, proving we could tackle more complex organisms. Just three years later, in 1995, the first free-living organism, Haemophilus influenzae (1.8 Mb), had its entire genome mapped. Unlike viruses, this bacterium contained all the genetic information needed for independent life, making its genome a far more complex puzzle. By 1996, the complete genome sequence of a eukaryote, S. cerevisiae, weighing in at 12.1 Mb, was finalized. These milestones were like climbing higher and higher peaks, each one giving us a broader view of the genomic landscape.
The Human Genome Project
The stage was set for the ultimate quest: sequencing the entire human genome. Launched in 1990, the Human Genome Project (HGP) was an unprecedented international collaboration, a scientific moonshot that brought together researchers from around the globe. Its ambitious goals were to map and sequence all 3 billion base pairs of human DNA, identify all human genes, and make this information publicly available.
The project was an enormous feat of global collaboration, demonstrating what humanity can achieve when it works together. While there were parallel private efforts, the HGP’s commitment to open-source data was a paradigm shift. It ensured that the fundamental blueprint of human life would be accessible to all, accelerating research worldwide and preventing the patenting of human genes.
The HGP was declared “finished” in 2003, more than two years ahead of schedule and under budget, with an astonishing accuracy rate of less than one error in 20,000 bases. This wasn’t just a scientific achievement; it was a revolution. It provided a foundational resource for biomedical studies, enabling researchers to identify genetic variations linked to diseases, understand biological processes, and develop new diagnostic and therapeutic strategies. The HGP truly paved the way for modern genomics, changing biology from a gene-by-gene study to a holistic, systems-level approach. It gave us the complete instruction manual, allowing us to begin truly understanding ourselves.
How We Read DNA: The Core Processes of a Genome Project
Imagine trying to read a book that’s billions of pages long, written in a four-letter alphabet (A, T, C, G), and then figuring out what each word and sentence means. That’s essentially what a genomics project does! It involves a sophisticated, multi-stage pipeline to open up the secrets hidden within our DNA.
First, we need to sequence the DNA – literally reading the order of the A’s, T’s, C’s, and G’s. Because current technology can’t read the entire genome from end to end, we have to break it into smaller, manageable pieces, read those pieces, and then put them back together like a giant, intricate jigsaw puzzle. This is called genome assembly. Finally, we need to make sense of the assembled sequence, finding genes and understanding their roles – a process known as genome annotation. All these steps are powered by bioinformatics, the discipline that uses computational tools to make sense of biological data.
Each step presents its own computational challenges, especially when dealing with the sheer volume of data. A single human genome, for instance, amounts to around 200GB of raw data! Processing thousands of such genomes requires robust, scalable infrastructure. A common starting point for reading DNA is shotgun sequencing, where the DNA is randomly chopped into fragments. Powerful computational tools then sort through these fragments to reconstruct the original sequence, a task made exponentially more difficult by the highly repetitive regions common in complex genomes.
DNA Sequencing Technologies
The ability to “read” DNA has evolved dramatically since Fred Sanger’s early methods. Today, we have a diverse toolkit of technologies, each with its own strengths and applications. Here’s a quick look at how the different generations of sequencing stack up:
| Technology | How it Works | Key Characteristics |
|---|---|---|
| Sanger Sequencing | Uses chain-terminating dideoxynucleotides to create fragments of different lengths, which are then separated by size to read the DNA sequence. It reads one DNA fragment at a time. | Gold-standard accuracy (>99.99%). Long reads (500-1000 bp). Low throughput, high cost per base. Ideal for validating specific gene sequences or small-scale projects. |
| Next-Generation Sequencing (NGS) | Massively parallel sequencing. Millions of DNA fragments are sequenced simultaneously on a flow cell. Each base is identified as it’s added to the growing DNA strand, often using fluorescent tags. | High throughput, low cost per base. Short reads (50-300 bp). Higher error rate than Sanger. Perfect for whole-genome (WGS) and whole-exome (WES) sequencing. |
| Third-Generation Sequencing | Sequences single DNA molecules in real-time without amplification. Technologies like PacBio (SMRT) and Oxford Nanopore detect base additions as a single DNA strand passes through a nanopore. | Very long reads (10,000-100,000+ bp). Can sequence in real-time. Higher error rate but excellent for resolving complex genomic regions and structural variants. Portability (e.g., MinION). |

