Genome Sequencing Methods That Changed Science Forever

Dna sequencing methods: 3 Revolutionary Generations
Why DNA Sequencing Methods Are the Foundation of Modern Biology
DNA sequencing methods are technologies that allow scientists to read the genetic code, determining the exact order of the four chemical building blocks—adenine (A), thymine (T), cytosine (C), and guanine (G)—that make up DNA.
The main DNA sequencing methods include:
- Sanger Sequencing – First-generation method using chain termination, highly accurate for small sequences
- Next-Generation Sequencing (NGS) – Second-generation methods that process millions of DNA fragments simultaneously
- Long-Read Sequencing – Third-generation methods like PacBio SMRT and Oxford Nanopore that read very long DNA sequences
The journey of DNA sequencing began with the first complete DNA genome—bacteriophage φX174—sequenced in 1977. Today, we can sequence the entire human genome with its 3 billion base pairs in just days for under $1,000, compared to the original Human Genome Project that took over a decade and cost billions of dollars.
This dramatic evolution has transformed everything from personalized medicine to agricultural breeding to forensic investigations. The ability to read genetic information quickly and affordably has opened doors to understanding diseases, tracking viral outbreaks, and even studying ancient DNA from extinct species.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in computational biology, my work focuses on making DNA sequencing methods more accessible to researchers worldwide, including contributions to the globally-used Nextflow workflow framework.
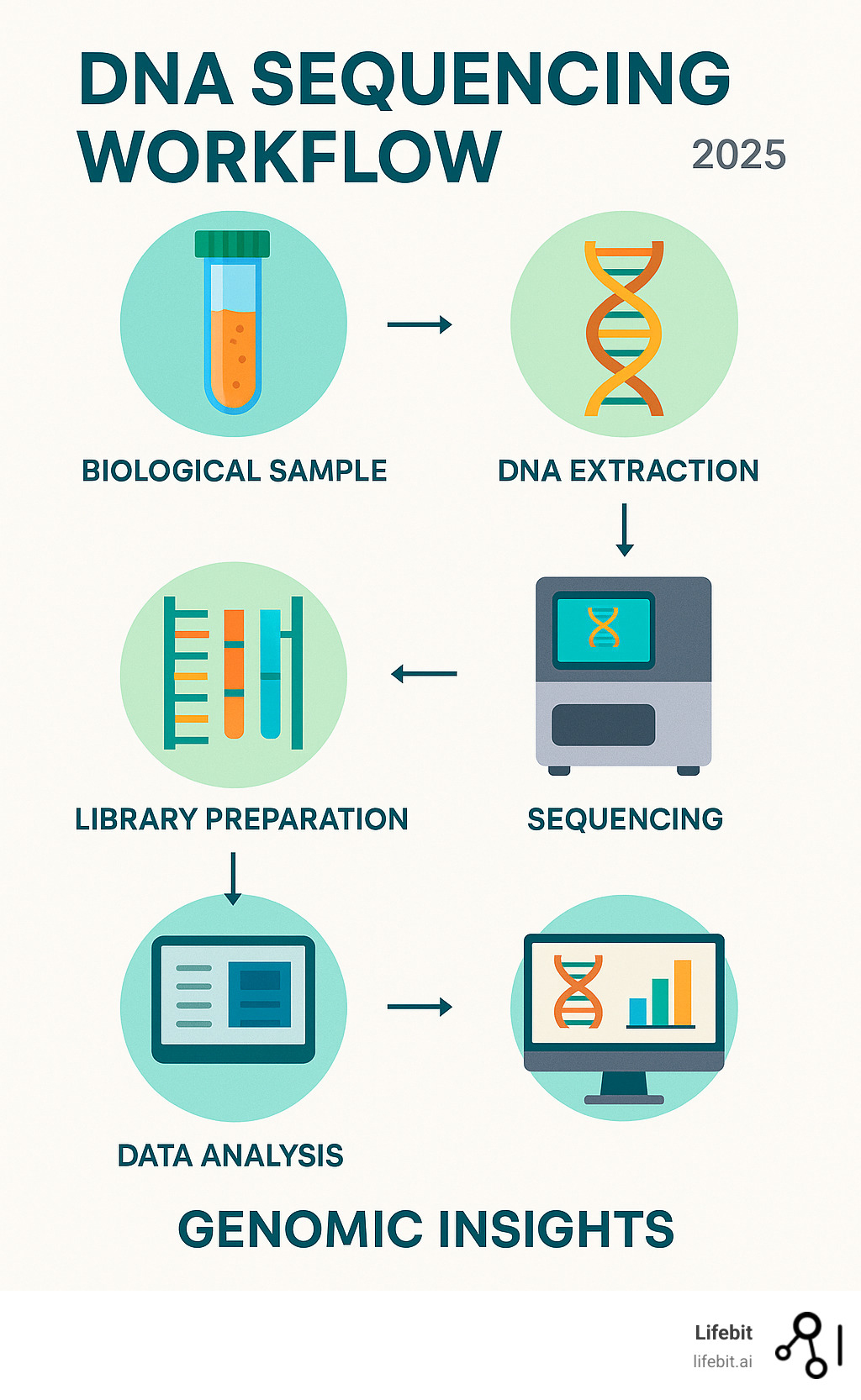
The Generations of DNA Sequencing Methods
Think of DNA sequencing methods as chapters in an incredible story of scientific findy. Each “generation” represents a major breakthrough that changed how we read the genetic code of life itself.
This journey began in the 1970s with two brilliant scientists who would forever change biology. Frederick Sanger and Walter Gilbert independently developed the first practical methods to read DNA sequences. Their work was so groundbreaking that both received Nobel Prizes – and Sanger actually won two Nobel Prizes for his contributions to sequencing both proteins and DNA.
The first major milestone came in 1977 when scientists successfully sequenced the complete genome of bacteriophage φX174. This tiny virus became the first organism to have its entire genetic blueprint decoded. By 1984, researchers had tackled something much larger – the Epstein-Barr virus with its impressive 172,282 nucleotides.
These early achievements laid the foundation for everything that followed. Today, we organize DNA sequencing methods into three main categories, each representing a quantum leap in our ability to read genetic information faster, cheaper, and more accurately.

First-Generation Sequencing: The Sanger Method Sets the Stage
When Frederick Sanger developed his chain-termination method in 1977, he created what would become the gold standard for DNA sequencing for decades. The method’s genius lies in its neat use of biochemistry to trick the cell’s own DNA replication machinery. The process begins with a primer, a short strand of DNA that binds to the template strand you want to sequence. Then, DNA polymerase, the enzyme that builds new DNA strands, is added along with all four standard nucleotide building blocks (dNTPs: dATP, dGTP, dCTP, dTTP). The key ingredient is a small amount of special modified building blocks called dideoxynucleotides (ddNTPs). These ddNTPs lack the specific hydroxyl group needed to form the next connection, so when the polymerase incorporates one, it acts as a definitive ‘stop sign,’ terminating DNA synthesis at that exact point.
Originally, this was done in four separate test tubes, each containing a different ddNTP (ddA, ddG, ddC, or ddT) labeled with a radioactive isotope. This created collections of DNA fragments of different lengths, all ending in a specific base. These fragments were then separated by size using gel electrophoresis on four parallel lanes, and the sequence was read manually from an X-ray film. A major innovation was the switch to fluorescent dyes, where each of the four ddNTPs was labeled with a different color. This allowed the entire reaction to happen in a single tube. Instead of cumbersome gels, the fragments are now separated by size using ultra-thin capillary electrophoresis, and a laser excites the dyes as they pass a detector. A computer then reads the sequence of colors to automatically decode the DNA sequence.
The high accuracy of Sanger sequencing (99.99%) and its ability to generate contiguous read lengths of up to 1,000 bases make it perfect for targeted sequencing of a single gene or region. However, its low throughput (one sequence at a time) and high cost per base make it impractical for large-scale projects like sequencing an entire genome. Despite this, it remains indispensable for plasmid sequencing, verifying the results of cloning, and for the crucial task of NGS result validation. Another first-generation method, Maxam-Gilbert sequencing, which used chemical degradation to cleave DNA at specific bases, is now largely obsolete due to its complexity and reliance on hazardous chemicals. Scientific research on the Sanger method continues to demonstrate its enduring value in specific applications.
Next-Generation Sequencing (NGS): Massively Parallel Power
The early 2000s brought a revolution with Next-Generation Sequencing (NGS), a paradigm shift that introduced massively parallel sequencing—the ability to read millions or even billions of DNA fragments simultaneously. This leap in scale is achieved through a combination of clever library preparation and innovative sequencing chemistry. The process begins with library preparation, where high-molecular-weight DNA is fragmented into smaller, manageable pieces. The ends of these fragments are repaired and an ‘A’ nucleotide is added to one end (A-tailing). Then, short, known DNA sequences called adapters are ligated onto both ends of the fragments. These adapters are crucial; they act as ‘handles’ that allow the fragments to bind to the surface of a flow cell, a glass slide where the sequencing reaction occurs.
The most dominant NGS technology, Illumina’s sequencing-by-synthesis (SBS), uses a process called bridge amplification. On the flow cell, each adapter-ligated fragment bends over and binds to a complementary adapter, forming a ‘bridge.’ A polymerase then synthesizes the reverse strand, creating a double-stranded bridge. This process is repeated over and over, resulting in a localized, dense cluster of millions of identical DNA fragments. Once the clusters are formed, the sequencing begins. Reversible dye-terminators are used, where each of the four nucleotides is labeled with a unique, removable fluorescent color and a temporary blocking group. In each cycle, the machine adds one nucleotide to the growing strand in every cluster. A high-resolution camera captures an image of the entire flow cell, recording the color emitted from each cluster. The blocking group and dye are then chemically cleaved, and the cycle repeats. This generates massive amounts of short-read data (typically 50-500 bases).
This high throughput approach generates enormous datasets at a remarkably lower cost per base, making large-scale projects like Whole-genome sequencing (WGS) and Exome sequencing routine. The main trade-off is the shorter read length, which can make it difficult to assemble complex genomes with repetitive regions. Furthermore, it requires a complex data analysis pipeline. Raw image files are converted into base calls (a process called base calling), reads are aligned to a reference genome, and sophisticated algorithms are used for variant calling and quality control. Despite these challenges, NGS has fundamentally transformed biology and medicine. For a deeper understanding of how NGS works, check out this overview of next generation sequencing.
Third-Generation Sequencing: The Dawn of Long Reads
While NGS brought high throughput, its short reads struggled with complex genomic regions like long repetitive sequences. This limitation paved the way for third-generation sequencing, also known as long-read sequencing. These technologies analyze single DNA molecules in real-time, often eliminating the need for PCR amplification, which can introduce biases. This allows them to generate reads stretching from 10,000 bases to over a million bases in length.
One leading technology is Pacific Biosciences’ (PacBio) Single-Molecule Real-Time (SMRT) sequencing. The magic happens inside tiny wells called Zero-Mode Waveguides (ZMWs), which are so small that they can only be illuminated at the very bottom where a single DNA polymerase enzyme is anchored. A single-stranded DNA template is fed through the polymerase. As the polymerase synthesizes the complementary strand, it incorporates fluorescently labeled nucleotides. Each of the four bases has a different colored dye attached to its phosphate tail. When a nucleotide is held by the polymerase during synthesis, it emits a flash of light that is recorded by a detector. The dye is then cleaved off with the phosphate group, leaving behind a natural DNA strand. This process allows for the real-time observation of DNA synthesis, molecule by molecule.
Another approach is Oxford Nanopore Technologies (ONT) sequencing. This method threads a single DNA molecule through a microscopic pore—a protein nanopore—embedded in a membrane. As the DNA passes through the pore, it disrupts an ionic current that is flowing through it. Each DNA base (A, T, C, G) has a unique shape and chemical structure, causing a characteristic disruption in the electrical signal. By measuring these changes in current, the system can decode the sequence of the DNA in real-time.
These very long reads are a game-changer. They can span entire repetitive regions and complex structural rearrangements, making it much easier to assemble genomes from scratch (de novo genome assembly) and to detect large structural variants (like insertions, deletions, and inversions) that are often missed by short-read methods. A key advantage is the ability to directly detect epigenetic modifications like methylation, as these modified bases produce a slightly different signal without any special sample preparation. This, combined with real-time analysis, is crucial for applications like rapidly tracking viral outbreaks. While early versions had higher error rates, modern systems like PacBio’s HiFi reads (which repeatedly sequence the same molecule to generate a highly accurate consensus) and improved ONT basecallers now achieve accuracy rivaling the gold standard, making long-read technologies increasingly powerful and accessible.
Head-to-Head: Comparing Key Sequencing Technologies
Choosing the right dna sequencing methods is a bit like picking the perfect tool from your toolbox – you wouldn’t use a wrench to hammer a nail, right? Each incredible technology we’ve discussed has its own special strengths and a few areas where it’s not the best fit. Understanding these differences is super important for anyone diving into genetics, whether you’re a seasoned researcher or a curious clinician.
Here, we’re going to put these powerful technologies side-by-side. We’ll look at key features like how long of a DNA piece they can read, how accurate they are, how much data they can churn out, and what the cost typically looks like. Think of this as your quick guide, a helpful “dashboard” of metrics to help you figure out which method will give you the most valuable information for your specific questions and budget.

Comparing First-Gen (Sanger), Second-Gen (NGS), and Third-Gen (Long-Read) sequencing
| Parameter | First-Generation (Sanger) | Second-Generation (NGS) | Third-Generation (Long-Read) |
|---|---|---|---|
| Read Length | Up to 1,000 bases | 50-500 bases (typically) | 10 kb to over 50 kb (can be megabases) |
| Accuracy | Very High (99.99%) | High (e.g., Illumina ~0.1% error) | Variable (historically lower, but improving; PacBio HiFi >99.9%, ONT up to 99.9%) |
| Throughput | Low (single fragment per run) | Very High (millions to billions of fragments per run) | High (single molecule, real-time) |
| Cost per Base | High | Very Low | Moderate to High (higher than NGS, but decreasing) |
| Key Advantage | High accuracy for targeted regions, confirmatory, relatively simple setup | Massively parallel, high throughput, extremely low cost per base for large-scale projects | Resolving complex genomic regions, de novo assembly, direct epigenetic detection, real-time analysis |
| Primary Applications | Targeted sequencing, variant confirmation, plasmid sequencing, low-throughput genotyping | Whole-genome sequencing (WGS), whole-exome sequencing (WES), RNA-Seq, ChIP-Seq, metagenomics, clinical diagnostics, cancer research | Structural variant detection, de novo genome assembly, epigenetic analysis, sequencing through repetitive regions, viral outbreak tracking |
As you can see from our comparison table, there isn’t one “best” dna sequencing method that rules them all. It truly comes down to what you’re trying to achieve! Your choice will depend on your specific research question, how complicated the DNA region you’re looking at is, how much data you need, and of course, your budget.
Often, the smartest approach involves using a combination of these methods. For example, you might use the massive power of NGS to get a broad overview of genetic changes, and then rely on Sanger for super precise validation of a specific finding. Or, you could use long-read sequencing to piece together tricky parts of a genome and then use short-read data for very accurate variant calling. It’s all about matching the tool to the task!
How DNA Sequencing is Revolutionizing Our World
Isn’t it amazing how something as tiny as a DNA molecule can hold so much power? The impact of dna sequencing methods truly stretches far beyond the quiet hum of research labs. These incredible technologies have woven themselves into the fabric of many different fields, sparking scientific breakthroughs and offering practical solutions to real-world challenges. From helping doctors personalize treatments to solving long-standing mysteries, the ability to read life’s genetic code is genuinely changing our world as we know it.

Changing Medicine with Advanced DNA sequencing methods
In medicine, dna sequencing methods are driving a shift toward preventative, personalized healthcare. In Personalized Medicine, a patient’s genetic code helps predict reactions to drugs (pharmacogenomics), minimizing side effects and maximizing efficacy. For Cancer Genomics, sequencing tumor DNA is essential for diagnosis, prognosis, and selecting targeted therapies. For rare genetic conditions, sequencing can end a long diagnostic odyssey. In Infectious Disease Management, it enables rapid pathogen identification, outbreak tracing, and monitoring of antibiotic resistance, as seen during the COVID-19 pandemic. Finally, in Reproductive Health, sequencing is used in prenatal testing to screen for genetic conditions, providing families with vital information. The applications are constantly expanding, as shown in this Research on DNA sequencing in cancer.
Open uping the Secrets of Biology and Evolution
Beyond medicine, dna sequencing methods are revealing profound secrets about life. The Human Genome Project, completed in 2022, provided the fundamental instruction manual for human life. In Evolutionary Biology, sequencing DNA from different species, including ancient DNA from extinct creatures, helps us build the family tree of life. Metagenomics allows us to sequence DNA directly from environmental samples (like soil or the gut) to understand microbial communities and their functions. This is crucial for Microbiome Studies, which link the trillions of microbes inside us to health and disease. Sequencing even helps us explore mysterious genomic regions like “Dark DNA,” which may hold important evolutionary clues. Paired with Transcriptomics and Proteomics Research, these methods allow us to decode biological processes on an unprecedented scale. Learn about ‘dark DNA’ and how it challenges our knowledge.
Powering Advances in Forensics, Agriculture, and Microbiology
The amazing versatility of dna sequencing methods extends into practical uses that affect our daily lives. In Forensic Science, DNA analysis is vital for identifying suspects, exonerating the innocent, and identifying human remains. For Agriculture and Food Safety, sequencing accelerates crop and livestock breeding by identifying genes for desirable traits like disease resistance. It also ensures food safety by tracking pathogens in outbreaks. In Microbiology and Environmental Protection, sequencing is crucial for monitoring ecosystems, tracking infectious diseases in animals, and identifying unknown viruses, with databases like GenBank holding millions of viral sequences.
Frequently Asked Questions About DNA Sequencing
We often get asked about the specifics of dna sequencing methods. It’s a truly fascinating field, and we’re happy to shed more light on some of the most common questions.
What is the most accurate of the dna sequencing methods?
Accuracy depends on the method and application. Sanger sequencing is the traditional ‘gold standard’ with ~99.99% accuracy for a single read, making it ideal for validating specific findings. Next-Generation Sequencing (NGS) platforms like Illumina achieve high consensus accuracy (typically 99.9%) by reading the same DNA stretch many times (‘high coverage’). Long-read sequencing has improved dramatically; PacBio’s HiFi reads and modern Oxford Nanopore technology now also exceed 99.9% accuracy. For the highest confidence, researchers often use a combination of methods.
How much does it cost to sequence a human genome?
The cost to sequence a human genome has plummeted. The original Human Genome Project cost billions. With the advent of Next-Generation Sequencing (NGS), the price fell dramatically. Today, the once-aspirational ‘$1000 genome‘ is a reality, with some services offering whole-genome sequencing for under $500. This incredible cost reduction has made genomics widely accessible for research, clinical diagnostics, and personal use.
What is the difference between DNA sequencing and genotyping?
Think of it as reading a whole book versus checking for specific keywords. DNA sequencing determines the exact order of every genetic letter (A, T, C, G) in a DNA segment. It provides a complete blueprint and can find novel, unknown genetic variations. Genotyping, in contrast, is a targeted test. It only checks for the presence of known genetic variants, like single nucleotide polymorphisms (SNPs), at specific, predefined locations in the genome. In short: sequencing is comprehensive, while genotyping is focused.
The Future of Genomics: What’s Next?
The world of dna sequencing methods is evolving at breakneck speed. The future isn’t just about faster, cheaper sequencing; it’s about revolutionizing how we analyze and act on genetic information.
Artificial intelligence and machine learning are becoming essential for making sense of the massive data from modern sequencers. AI excels at identifying genetic variants, assembling genomes, and finding complex patterns that humans might miss. The next leap is multi-omics integration, combining DNA data with information from RNA, proteins, and metabolites to get a complete biological picture. Furthermore, portability is now a reality, with handheld devices like the Oxford Nanopore MinION enabling sequencing in the field, far from a traditional lab.
However, as genomic datasets grow and spread globally, the greatest challenges are federated data analysis and data security and governance. We need ways to analyze this treasure trove of information while keeping it secure and private. This is precisely the challenge that drives our work at Lifebit.
Our next-generation federated AI platform tackles this head-on by enabling secure, real-time access to global biomedical and multi-omic data. We’ve built components like our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) specifically to deliver real-time insights while maintaining the highest standards of data security and compliance. This means researchers can collaborate across continents, pharmaceutical companies can conduct large-scale studies, and public health agencies can monitor disease patterns – all while ensuring sensitive genetic information stays protected.
The future of dna sequencing methods is bright, fast, and incredibly exciting. We’re heading toward a world where genetic insights are available instantly, globally, and securely. The potential to transform medicine, agriculture, and our understanding of life itself has never been greater. More info about Lifebit’s federated data platform.

