The AI Rx: Unlocking New Cures with Artificial Intelligence
AI-powered drug discovery: 3 Ways to Revolutionize Cures
The Urgent Need for a Pharmaceutical Revolution
AI-powered drug findy is revolutionizing the development of life-saving medicines by using artificial intelligence to dramatically reduce the time, cost, and failure rates of traditional pharmaceutical research. Here’s what you need to know:
Key Benefits of AI-Powered Drug Findy:
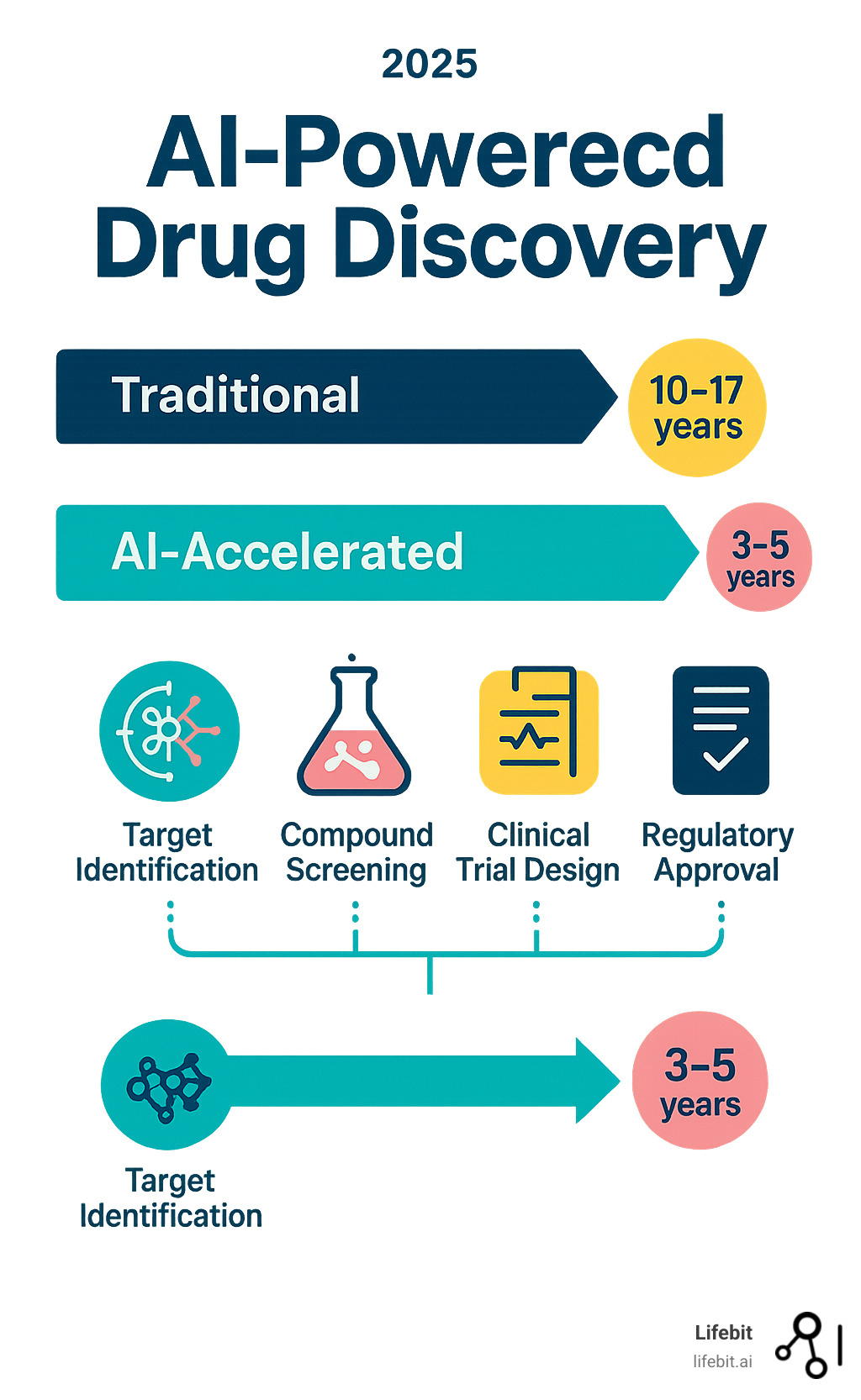
- Reduced Timeline: From 10-17 years down to potentially 3-5 years
- Lower Costs: Traditional development costs $1-2 billion per drug
- Higher Success Rates: AI can predict failures early, improving the current 5% clinical success rate
- Novel Target Findy: AI identifies previously unknown disease targets
- Accelerated Clinical Trials: Better patient matching and outcome prediction
The pharmaceutical industry faces an urgent crisis. Developing a single new drug takes 10 to 17 years and costs between $1-2 billion, with a staggering 95% failure rate in clinical trials. This broken system means patients wait decades for treatments that may never come. For the roughly 7,000 rare diseases, treatments have been found for only 500 in over a century of modern medicine.
But change is coming fast. AI has the potential to increase pharmaceutical industry returns by more than 45% through strategic investment. For example, some drug findy challenges have been completed in just 21 days using AI, and AI-finded treatments have advanced to Phase II trials in only 18 months.
The revolution isn’t theoretical – it’s happening now, with fully AI-generated drugs entering Phase IIa trials as of 2024.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where I’ve spent over 15 years developing computational biology and AI platforms that power AI-powered drug findy across secure, federated environments. My work spans from contributing to breakthrough genomics tools like Nextflow to building platforms that enable pharmaceutical organizations to open up insights from siloed biomedical data.
The Core Engines: Understanding AI in Pharmaceutical R&D
AI-powered drug findy relies on three core components: data, computation, and algorithms. These have been changing pharmaceutical science for decades, but recent advancements in all three areas have converged to open up their full potential.
- Artificial Intelligence (AI) is the broad field of creating intelligent machines capable of performing tasks that typically require human intelligence.
- Machine Learning (ML), a subset of AI, enables systems to learn patterns and make predictions from data without being explicitly programmed. Instead of following a fixed set of rules, ML models adjust their internal parameters based on the data they are trained on.
- Deep Learning (DL), a specialized type of ML, uses multi-layered neural networks inspired by the human brain’s structure. These networks, containing many layers of interconnected nodes, can automatically learn and represent highly complex, hierarchical relationships in vast datasets. This makes them particularly powerful for biological data. For example, Convolutional Neural Networks (CNNs) are adept at analyzing image data from microscopy or histopathology slides, while Recurrent Neural Networks (RNNs) excel at processing sequential data like genomic (DNA/RNA) or proteomic (amino acid) sequences.
- Natural Language Processing (NLP) is a branch of AI that allows machines to read, understand, interpret, and generate human language. In drug findy, NLP can instantly scan and synthesize information from millions of scientific papers, patents, and clinical trial reports to identify novel drug targets or potential side effects.
- Generative AI is the creative engine of modern AI. This subfield, which includes models like Generative Adversarial Networks (GANs), Variational Autoencoders (VAEs), and Transformers, is capable of designing entirely new molecular structures, proteins, or gene sequences that have never existed before but possess specific, desirable properties.
While AI has been applied in pharmaceuticals since the 1990s, only recently has the combination of massive datasets, powerful computing hardware (like GPUs), and mature algorithms allowed the technology to deliver today’s breakthrough results.
How AI Learns from Biomedical Data
AI requires vast amounts of diverse, high-quality biomedical data to learn the complex rules of biology and chemistry. Key sources include:
- Genomics data: Provides the complete DNA sequence of an organism or individual. This helps AI link specific genetic variations to disease susceptibility, progression, and response to drugs, forming the foundation of personalized medicine.
- Transcriptomics data: Measures gene expression (e.g., via RNA sequencing), revealing which genes are active or inactive in a cell at a given time. This dynamic data helps AI understand how cells respond to disease or treatment.
- Proteomics data: Focuses on proteins, the functional workhorses of the cell and the targets for most drugs. Understanding protein structure, function, and interaction networks is crucial for designing effective treatments.
- Metabolomics data: Analyzes the small molecules (metabolites) within cells and biological systems. This provides a real-time snapshot of cellular physiology and can reveal biomarkers for disease or drug toxicity.
- Clinical trial data: Offers real-world evidence from past studies, teaching AI about drug efficacy, safety profiles, and patient outcomes across different populations.
- Electronic Health Records (EHRs) and Real-World Data (RWD): Contain rich, longitudinal patient information that helps AI understand how diseases manifest and how drugs perform outside of the controlled setting of a clinical trial. This includes data from sources like patient registries and even wearable devices.
- Multi-omics integration: The true power of AI is unleashed when it combines these different data types. By integrating genomics, proteomics, transcriptomics, and other data, AI can build a holistic, systems-level view of biology, uncovering relationships that would be invisible within a single data type.
Through pattern recognition, AI spots intricate, non-linear relationships in this data that humans might miss. This fuels predictive modeling, allowing us to forecast a drug’s properties, a patient’s response, or potential side effects before costly and time-consuming experiments begin.
At Lifebit, we know that this valuable data is often trapped in organizational or geographical silos. That’s why robust data standardization, harmonization, and federated technologies are essential for effective AI-powered drug findy at scale.
Key AI Algorithms Driving Findy
Several sophisticated AI algorithms work in concert to drive drug findy:
- Supervised learning uses labeled data to make predictions. For example, a model can be trained on a dataset of molecules with known toxicity levels (the labels) to learn to predict whether a new, unseen molecule will be toxic.
- Unsupervised learning finds hidden patterns and structures in unlabeled data. This is useful for tasks like clustering patients into distinct subgroups based on their molecular profiles (patient stratification) or grouping molecules by structural similarity.
- Reinforcement learning trains AI agents to make optimal decisions through trial and error. The agent receives ‘rewards’ or ‘penalties’ for its actions, learning a strategy over time. This has proven powerful for de novo drug design, where the AI agent is rewarded for designing molecules that have better binding affinity and drug-like properties.
- Graph Neural Networks (GNNs) are a cutting-edge approach that represents molecules as graphs (with atoms as nodes and bonds as edges). This allows the AI to learn directly from the molecule’s 2D or 3D structure, capturing complex topological and chemical relationships to better predict properties like solubility and binding affinity.
- Generative models, including Generative Adversarial Networks (GANs), Variational Autoencoders (VAEs), and Transformer-based models, are the creative force behind AI drug findy. GANs use a two-part system (a generator and a discriminator) to create novel yet realistic molecules. VAEs learn a compressed representation of known molecules and then generate new ones from that learned ‘latent space’. Transformers, originally developed for language, excel at learning the ‘grammar’ of chemical structures to build new molecules piece by piece. These models move us beyond screening existing libraries to designing the perfect molecule from scratch.
How AI-Powered Drug Findy is Changing the Pipeline

The traditional drug findy pipeline is a long, linear, and arduous process. AI-powered drug findy is reshaping every stage, from initial concept to final validation, by introducing iterative, data-driven cycles that accelerate timelines, reduce costs, and improve success rates.
- Target Identification: Pinpointing biological molecules (e.g., proteins, genes) that are causally involved in a disease.
- Hit Identification: Finding initial molecules (‘hits’) that interact with the chosen target.
- Lead Optimization: Chemically modifying hit compounds to improve their potency, selectivity, and drug-like properties, turning them into ‘leads’.
- Preclinical Studies: Testing lead compounds in laboratory (in vitro) and animal (in vivo) models to assess safety and efficacy.
- Clinical Trials: Testing in humans across multiple phases (I, II, III) to assess safety, dosage, and effectiveness.
- Drug Repositioning: Finding new therapeutic uses for existing, approved drugs.
Revolutionizing Target Identification and Validation
Identifying the right biological target is the critical first step; a mistake here dooms the entire project. AI revolutionizes this stage by analyzing vast genomic and multi-omic datasets to distinguish correlation from causation, uncovering novel targets and predictive biomarkers. Natural Language Processing (NLP) complements this by mining millions of scientific literature articles, patents, and clinical trial databases for disease-relevant pathways and gene-disease associations. This information is then integrated into biomedical knowledge graphs, which are massive networks connecting genes, proteins, diseases, compounds, and clinical outcomes. AI algorithms can then traverse these graphs to identify non-obvious relationships and propose novel, high-confidence targets that might have been missed by human researchers. For a deeper dive, we recommend this Scientific review on AI in drug development.
Case Study: AI-driven Rare Disease Target Findy
AI has shown immense power in rare disease research, where data is scarce. For example, an AI platform was used to analyze genetic and clinical data to identify novel targets and screen candidate compounds for Wilson’s disease, a rare genetic disorder. The entire process from target hypothesis to lead candidate identification was completed in just 18 months—a remarkable acceleration compared to the years it would traditionally take. This demonstrates how AI can increase our chances of success in areas with high unmet medical needs.
De Novo Drug Design and Virtual Screening
Once a target is identified, AI accelerates the design of molecules that can interact with it. Generative models can design entirely new molecules (de novo design) from scratch, optimized for desired properties like high binding affinity to the target and low affinity for other proteins to minimize side effects. AI also supercharges virtual screening of existing compound libraries:
- Structure-Based Virtual Screening (SBVS): When the 3D structure of the target protein is known, deep learning models can predict the binding affinity between the target and millions of potential drug candidates far more accurately and quickly than traditional physics-based docking methods.
- Ligand-Based Virtual Screening (LBVS): When the target structure is unknown, AI can use methods like Graph Neural Networks to build quantitative structure-activity relationship (QSAR) models. These models learn from a set of known active molecules to find other compounds with similar structural or chemical features.
Crucially, AI platforms can now predict a compound’s ADMET properties (Absorption, Distribution, Metabolism, Excretion, and Toxicity) at the earliest stages. Failures due to poor ADMET profiles are a major reason why over 90% of drugs fail in clinical trials. By predicting these properties in silico, AI allows chemists to filter out or redesign molecules with likely ADMET issues long before they enter costly preclinical testing. A groundbreaking advancement is AI’s ability to predict protein structures. For example, DeepMind’s AlphaFold and its successor, AlphaFold 3, have achieved incredible accuracy in predicting the 3D structures of proteins and their interactions with other molecules like DNA and RNA. This capability is invaluable for structure-based drug design, especially for targets whose structures have not been solved experimentally. Learn more in this Research on AI for protein structure prediction.
Case Study: AI-accelerated Candidate Identification
The impact of AI is clear and tangible. In March 2024, a fully AI-generated drug for idiopathic pulmonary fibrosis (IPF) entered Phase IIa trials, a major milestone demonstrating that AI can create viable clinical candidates. This follows earlier successes, such as an AI-designed molecule for obsessive-compulsive disorder entering clinical trials in 2020 and another AI platform identifying novel hits for hundreds of targets, proving its viability as a scalable alternative to expensive, slow high-throughput screening.
Accelerating and De-risking Clinical Trials
Clinical trials are the most expensive, time-consuming, and riskiest phase of drug development. AI is making this phase more efficient and predictive in several ways:
- Intelligent Trial Design: AI can optimize trial protocols by predicting the most effective endpoints, duration, and dosage regimens. It can also create in silico clinical trials (ISCT) by building ‘digital twins’ or virtual patient cohorts from aggregated clinical and omics data. These simulations allow researchers to test hypotheses and refine inclusion/exclusion criteria before enrolling a single patient, reducing the risk of costly trial failures.
- Patient Stratification and Recruitment: AI algorithms can analyze electronic health records (EHRs) and genomic data to identify and stratify patients who are most likely to respond to a treatment. This precision approach leads to smaller, faster, and more successful trials. AI-powered platforms can then match these eligible patients to suitable trials with significantly higher precision and speed than manual methods.
- Trial Monitoring and Adherence: During a trial, AI can be used to monitor patient adherence to the treatment protocol through digital tools and wearables. For instance, AI-powered algorithms can verify patient adherence through video or sensor data, with studies showing adherence increasing from a typical 50% to over 90%, improving data quality.
- Drug Repositioning: Finding new uses for existing drugs—is another powerful application. Because these drugs have already passed safety testing, their path to approval for a new indication is much faster and cheaper. AI facilitates this by mining multi-omic data, scientific literature, and clinical records to find novel connections between a drug’s mechanism and a different disease’s pathology. For example, AI was used to identify a new treatment for fragile X syndrome, advancing it to Phase II trials within 18 months. AI has also been used to explore repurposed drugs for Alzheimer’s Disease and famously identified baricitinib as a potential treatment for severe COVID-19.
Case Study: AI-enabled Clinical Trial Optimization
The journey of an AI-generated drug for idiopathic pulmonary fibrosis (INS018_055 by Insilico Medicine) to Phase IIa trials in March 2024 is a testament to AI’s power to compress the entire R&D timeline. This, along with the rapid advancement of a repurposed drug for fragile X syndrome to Phase II, underscores AI’s ability to not only discover new drugs but also to accelerate their clinical development and validation.
Navigating the Problems: Challenges and Solutions in AI Implementation

Implementing AI-powered drug findy is not without its hurdles. These are not insurmountable roadblocks but rather critical challenges that the industry is actively working to solve. Addressing them is key to unlocking AI’s full potential.
The biggest problems include:
- Data Quality and Standardization: AI models are only as good as their data. Biomedical data is notoriously noisy, heterogeneous, and often suffers from batch effects (technical variations from different experimental runs) and publication bias (where negative or null results are underreported), skewing the data AI learns from. Furthermore, data from different sources often lacks standardization in formats and terminology, requiring intensive and costly data cleaning and harmonization efforts.
- Data Silos and Privacy: Highly valuable proprietary and patient-level datasets are often isolated within individual organizations or hospitals due to privacy concerns and competitive interests. This prevents the large-scale data aggregation that makes AI so powerful.
- Model Interpretability and Trust: Many advanced AI models, particularly in deep learning, operate as “black boxes,” making it difficult for scientists and regulators to understand or trust their predictions. If a model suggests a novel drug target, researchers need to understand the biological rationale behind the prediction.
- Ethical, IP, and Bias Issues: Complex questions around ownership of AI-generated discoveries are emerging. If an AI invents a drug, who is the inventor? The AI’s developer, the owner of the training data, or the user? Additionally, if training data is not diverse, AI models can perpetuate and even amplify existing health disparities, creating drugs that are less effective for underrepresented populations.
- Regulatory Approval: Regulatory bodies like the FDA and EMA are still developing and refining frameworks for evaluating drugs and diagnostics developed using AI. This creates uncertainty for companies about the validation and documentation required for approval.
The Challenge of Data and Model Interpretability
Data privacy and security are non-negotiable. Federated learning is a key technological solution that is gaining traction. It allows AI models to be trained on distributed data sources without the raw, confidential data ever leaving its secure environment. Instead, the model ‘travels’ to the data, learns from it locally, and only a non-sensitive model update is shared. The EU-funded Melloddy project successfully demonstrated how 10 rival pharmaceutical companies could collaboratively train a predictive model on their combined proprietary datasets while protecting their individual intellectual property.
To tackle the “black box” problem, the field of Explainable AI (XAI) is crucial. XAI provides techniques to make AI predictions transparent and understandable. Methods like SHAP (SHapley Additive exPlanations) and LIME (Local Interpretable Model-agnostic Explanations) can highlight which features in the data (e.g., which specific genes or molecular substructures) were most influential in a model’s decision. This is vital for building trust, allowing scientists to validate AI outputs against biological knowledge, and providing a mechanistic rationale for regulatory submissions. XAI also helps identify and correct algorithmic bias, ensuring that AI-powered drug findy benefits everyone equitably. Without explainable biology, AI systems are just sophisticated guessing machines; real breakthroughs require AI that scientists can interrogate, understand, and trust.
Integrating AI with Traditional Wet Lab Experiments
Successful AI-powered drug findy does not replace wet lab experiments; it makes them smarter, faster, and more targeted. This iterative “lab-in-the-loop” or “self-driving lab” approach creates a continuous improvement cycle that combines computational power with real-world validation.
- AI Prediction: The AI model analyzes data and generates hypotheses, such as predicting the top 10 most promising compounds to synthesize or identifying a key protein to test.
- Optimized Experimentation: The AI then helps design the most efficient experiment to validate these hypotheses, minimizing the use of time, reagents, and resources.
- Automated Execution: Automated labs with robotic systems can then execute these AI-generated plans, running assays, synthesizing compounds, and collecting data with high throughput and reproducibility.
- Feedback Loop: The new experimental results are fed back into the AI model, which learns from the outcomes and refines its next round of predictions.
This creates human-in-the-loop models, where AI amplifies human expertise, not replaces it. Scientists are freed from tedious, repetitive tasks to focus on high-level strategy, creative problem-solving, and interpreting complex results. The integration of wet lab experiments and computational work combines the speed and scale of AI with the rigor of empirical science, leading to more robust and reliable drug development.
Frequently Asked Questions about AI in Drug Findy
How does AI actually speed up the drug findy process?
AI-powered drug findy acts as a brilliant, tireless research assistant that automates and accelerates complex analysis at a massive scale. Instead of relying on slow, expensive, and often serendipitous trial-and-error, researchers can use AI to intelligently navigate the vast chemical space. AI systems can predict a molecule’s behavior—how strongly it will bind to a target, its likely side effects, its solubility, and how easily it can be synthesized—allowing researchers to focus only on the most promising candidates.
Through pattern recognition, AI spots subtle, multi-dimensional relationships in biological data that are impossible for humans to see. For example, it can identify a common genetic signature among patients who respond well to a certain class of drugs. It can also generate synthesis pathways for novel compounds, creating a step-by-step chemical ‘recipe’ for how to make them in the lab. This transforms years of painstaking work into weeks or months of highly targeted, hypothesis-driven research, dramatically reducing the time from initial idea to a viable drug candidate.
What are some of the biggest successes of AI in drug findy so far?
We’re already seeing breakthrough moments that once seemed like science fiction, moving from theoretical promise to real-world clinical impact.
Protein structure prediction has been fundamentally transformed by tools like DeepMind’s AlphaFold. Before AlphaFold, experimentally determining a single protein’s 3D shape could take years; now, AI can predict the structures of hundreds of thousands of proteins with high accuracy in a short time. This is crucial for designing drugs that fit their targets perfectly, a cornerstone of structure-based drug design.
Real AI-designed drugs are now in human clinical trials. For example, Insilico Medicine’s INS018_055, a drug for Idiopathic Pulmonary Fibrosis (IPF) with a novel target and molecular structure both discovered by AI, entered Phase IIa trials in 2024. Another AI-guided treatment for fragile X syndrome reached Phase II trials in just 18 months, a fraction of the typical time.
Drug repositioning has also seen major wins. During the COVID-19 pandemic, AI helped identify baricitinib, an existing arthritis drug, as a potential treatment for severe cases by analyzing the drug’s mechanism of action against the disease’s inflammatory response. In antibiotic findy, a critical area given the rise of antimicrobial resistance, AI was used to discover halicin. By screening a library of compounds for activity against E. coli, an AI model identified this novel molecule which was later shown to be effective against numerous dangerous, drug-resistant bacteria, including Clostridioides difficile and Acinetobacter baumannii.
Is AI going to replace human scientists in the pharmaceutical industry?
No. AI-powered drug findy is about amplification, not replacement. It’s a powerful tool that excels at processing vast amounts of data, recognizing patterns, and performing repetitive tasks, but it cannot replicate the creativity, intuition, strategic thinking, and ethical judgment of human scientists. The goal is to create a synergy where AI and humans each do what they do best.
Human expertise becomes more, not less, valuable. With AI handling the data-heavy lifting, scientists can focus on higher-level problem-solving: asking the right biological questions, designing clever experiments, and interpreting the nuanced results that AI provides. The most successful teams use a human-in-the-loop approach. For example, an AI might generate 1,000 novel molecular structures; a medicinal chemist then uses their expertise to select the 20 most promising candidates based on synthetic feasibility and potential off-target effects. This expert feedback is then used to retrain the AI for the next design cycle.
By freeing researchers from tedious analysis, AI fosters creativity and allows them to ask bigger, more complex questions about human health. The future is a close collaboration between human experts and intelligent AI systems to solve medicine’s greatest challenges faster and more effectively.
Conclusion: A New Era of Precision and Speed in Medicine
AI-powered drug findy has moved from science fiction to reality, fundamentally changing how we approach medicine. We are shifting from a world where drug development takes over a decade and costs billions to one where AI can identify promising compounds in weeks.
The goal is not just to reduce costs and timelines but to increase success rates. By working smarter, AI helps predict failures early, saving resources and getting effective treatments to patients faster. This revolution is paving the way for truly personalized medicine, with treatments custom to an individual’s unique biological makeup.
The future of personalized medicine depends on our ability to securely access and analyze global biomedical data. This requires integrated platforms that can harmonize data from multiple sources, apply advanced AI, and maintain strict security and governance. Success in AI-powered drug findy happens through collaboration, not in isolation.
At Lifebit, we’ve built this exact federated AI technology. Our platform enables pharmaceutical companies, research institutions, and public health agencies to collaborate on drug findy without compromising data privacy. Our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) work together to deliver the real-time insights that make breakthroughs possible.
This technology enables secure collaboration across hybrid data ecosystems, allowing researchers to access global datasets and share insights while maintaining full control over their proprietary information. With AI as our partner and federated platforms enabling secure collaboration, we are entering an era where precision medicine is becoming a reality, bringing the right treatment to the right patient at the right time.


