Unlocking Patient Insights with Clinical Registry Software
Clinical Registry Software: Top 7 Features
Why Clinical Registry Software is Changing Healthcare Data Management
Clinical registry software is a specialized platform for collecting, managing, and analyzing patient data over time. As the backbone of evidence-based medicine, these systems help improve outcomes, support research, and ensure treatment safety by tracking patient populations and generating real-world evidence.
Key capabilities include automated data collection, advanced analytics, regulatory compliance (HIPAA, GDPR), seamless system integration, and patient engagement tools like ePROs.
The market is expanding rapidly, projected to grow from $1.72 billion in 2024 to $3.12 billion by 2029, a 12.53% CAGR. This reflects an urgent need for better data management. The impact is clear: registry data has helped drive a 30% reduction in post-surgical opioid prescribing and a 24% decrease in postoperative mortality in certain studies, changing manual processes into automated workflows.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years developing biomedical data platforms, I’ve seen how the right clinical registry software can open up transformative patient insights for organizations worldwide.
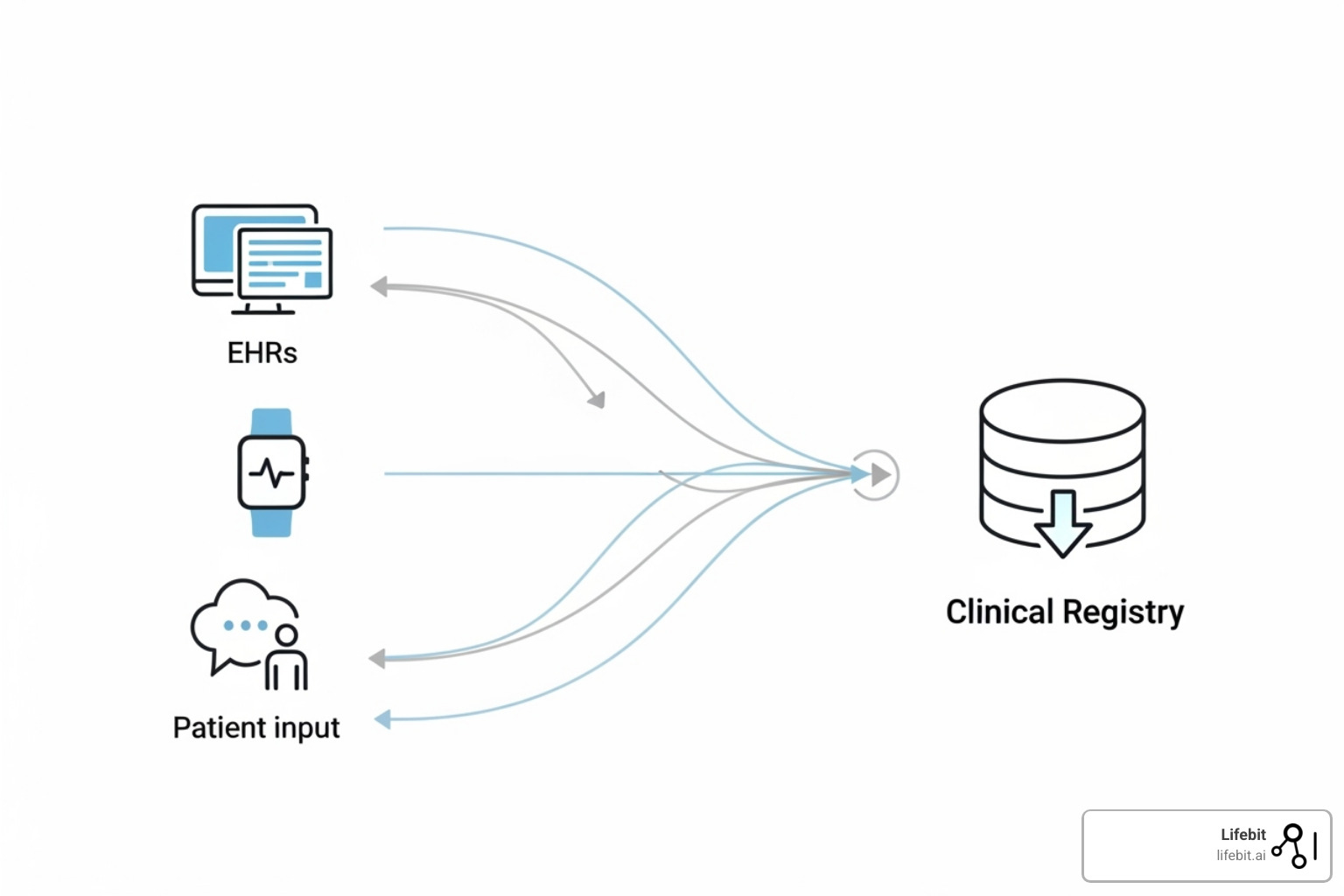
Basic clinical registry software terms:
What is a Clinical Registry and Why is It Crucial for Modern Healthcare?
A clinical registry is an organized system that uses observational methods to collect and analyze healthcare information over time. It acts as the collective memory of healthcare, providing a long-term view of how conditions develop, how they are managed, and what the real-world results are. This is essential for evidence-based medicine and for post-market surveillance of drugs and devices.
Modern registry platforms have evolved from simple databases into advanced analytical tools that turn raw data into actionable insights, leading to more efficient care and better patient outcomes. For a deeper dive, you can read our article on What Are Patient Registries, Why Are They Important?.
The Primary Purpose of Patient Registries
Patient registries are active tools designed to:
- Track outcomes: Monitor the effectiveness and safety of treatments in real-world settings.
- Understand disease progression: Map the natural course of diseases, especially rare ones.
- Assess treatment safety: Identify long-term side effects of drugs and devices through post-market surveillance.
- Evaluate care quality: Benchmark provider performance to highlight best practices.
- Support clinical research: Provide rich real-world data (RWD) for observational studies and trial recruitment.
- Facilitate public health surveillance: Monitor disease prevalence and track public health initiatives.
The Transformative Impact on Care Quality
Data from clinical registries translates directly into improved patient care. By enabling performance benchmarking, identifying care gaps, and helping develop clinical guidelines, these platforms drive continuous improvement.
The impact is measurable and can be life-saving. For example, a pediatric cardiac consortium used registry data to achieve a remarkable 24% decrease in postoperative mortality. In another case, a surgical collaborative used registry insights to achieve a 30% drop in opioid prescribing without affecting patient satisfaction.
These examples show how clinical registry software empowers organizations to make data-driven decisions that lead to tangible improvements in care quality, patient safety, and overall outcomes.
A Guide to the 5 Main Types of Clinical Registries
Clinical registries are diverse, each designed to focus on a particular area of health data. While all aim to improve patient outcomes, they differ in their data focus, research goals, and stakeholders. Understanding these types highlights the broad applications of clinical registry software, which is not a one-size-fits-all solution.

From studying rare conditions to monitoring medical devices, each registry type is vital. At Lifebit, our clinical registry software is built with flexibility at its core, adapting to the unique data collection, analysis, and reporting needs of any registry type.
1. Disease Registries
Disease registries collect detailed, longitudinal information on patients diagnosed with a specific illness. They are crucial for understanding the natural history of disease by tracking how conditions progress, respond to treatments, and impact patients over time, which is especially important for chronic and progressive illnesses.
For rare diseases, where patients are geographically scattered and clinical trial recruitment is difficult, these registries are a lifeline. They pool data from disparate sources to create a cohort large enough for meaningful research, accelerating the development of new therapies. A prime example is the Cystic Fibrosis Foundation Patient Registry, which has been instrumental in tracking the health of individuals with CF and has contributed to a dramatic increase in life expectancy.
These registries also empower patients by systematically capturing patient-reported outcomes (PROs). Modern clinical registry software facilitates this through integrated patient portals or mobile applications, allowing patients to report on their quality of life, symptoms, and functional status directly. This provides a complete, 360-degree view of the patient experience that is often missed in clinical data alone. For example, a registry for a serious lung disease brought together data from thousands of patients across dozens of sites, leading to numerous conference presentations and research papers. This demonstrates the immense scientific value generated by a dedicated disease registry.
Our clinical registry software is designed to support the complex data collection (including genomic and imaging data), longitudinal tracking, and advanced analytical needs of these specialized Disease Registers.
2. Product or Medical Device Registries
Product or Medical Device Registries are specialized systems that track the performance, safety, and effectiveness of specific medical products—such as pharmaceuticals, biologics, or devices—after they enter the market. They are essential for post-market surveillance, a requirement mandated by regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These registries provide long-term, real-world data that complements the findings from controlled, pre-market clinical trials, helping to identify rare side effects or long-term complications that may not appear in shorter studies.
These registries are critical for device performance monitoring, especially for high-risk implants like pacemakers, artificial joints, or heart valves. They track function over a device’s entire lifespan to spot design flaws, material degradation, or premature failures. This continuous monitoring directly improves patient safety by enabling timely alerts, recalls, and updates to clinical practice. For instance, the STS/ACC TVT Registry™ tracks patients undergoing transcatheter valve therapies, providing vital real-world information on procedural safety and long-term device effectiveness.
Manufacturers often lead or sponsor these registries to gather additional evidence for label expansion, explore new product uses, or fulfill regulatory obligations. The data generated creates a crucial feedback loop, informing next-generation product design and refining clinical guidelines for use. This highlights how clinical registry software is fundamental to ensuring the ongoing safety and efficacy of medical innovations.
3. Health Service or Specialty Registries
Created and managed by medical specialty organizations, health service or specialty registries focus on improving the quality and value of care within a specific medical field, such as cardiology, oncology, or orthopedics. They assess the quality of care by collecting highly detailed, procedure-specific data on patient characteristics, processes of care, and outcomes.
A key function is benchmarking, which allows hospitals and individual providers to compare their risk-adjusted performance against aggregated national or regional data. This isn’t just about comparing raw numbers; sophisticated clinical registry software uses statistical models to account for differences in patient populations, ensuring a fair and accurate comparison. This fosters a culture of continuous improvement by highlighting top performers and identifying areas for growth. For example, the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) is a leading surgical registry that has been shown to reduce complications and improve surgical outcomes for participating hospitals.
The insights gathered are also invaluable for guideline development, ensuring clinical practices are aligned with the latest real-world evidence. These registries facilitate highly focused research within a specialty and support quality improvement cycles (like Plan-Do-Study-Act), where institutions can implement changes and use the registry to measure their impact. Our clinical registry software is designed to support these specialized, high-impact registries and help lift the standard of care across entire medical fields.
4. Population-Based Registries
Unlike disease-specific or hospital-based registries, population-based registries capture health information from entire populations within a defined geographic area (e.g., a country, state, or city) or a specific demographic group, regardless of health status or where they receive care. This comprehensive population scope minimizes the selection bias inherent in registries that only draw from specific hospitals, making them exceptionally powerful tools for public health.
They are fundamental for monitoring public health trends and conducting epidemiological research that shapes health policy. A classic example is national cancer registries, such as the Surveillance, Epidemiology, and End Results (SEER) Program in the United States. SEER collects data on cancer incidence, prevalence, mortality, and survival rates, providing critical insights into the nation’s cancer burden. This data is used to identify health disparities among different populations, evaluate the effectiveness of screening programs, and guide resource allocation.
Clinical registry software for these studies must be built for massive scale and data diversity. These registries provide the evidence needed for crucial resource allocation decisions, such as where to build new clinics or which communities need targeted screening programs. For example, a registry established during the COVID-19 pandemic rapidly enrolled tens of thousands of healthcare workers and community members across the U.S. It pivoted to track vaccine outcomes, demonstrating how these platforms can provide critical real-world evidence during public health emergencies. This ability to follow large, diverse populations over time is essential for understanding community health and informing policy.
5. Payer Registries
Payer registries are used by insurance companies, government health programs (like Medicare and Medicaid), and other payers to measure and improve the value of care delivered to their members. Organized around covered populations rather than specific diseases or providers, these registries are central to the shift towards value-based care initiatives that reward quality outcomes and efficiency over service volume.
They are crucial for cost-effectiveness analysis, helping payers identify which treatments, providers, and care pathways deliver the best outcomes for a reasonable cost. This is achieved by integrating clinical data with administrative claims data to get a full picture of the care journey. They also enable population health management by analyzing data to understand the health risks and needs of covered populations, allowing for targeted preventive programs (e.g., for diabetes management or smoking cessation) that can reduce long-term costs.
Payers use insights from these registries to design benefit plans, create tiered provider networks, and negotiate performance-based contracts with healthcare systems. The ultimate goal is tracking member outcomes to ensure high-quality, effective care. For clinical registry software platforms, supporting payer registries requires handling massive, secure datasets and bridging the gap between clinical and financial data to turn complex information into insights that benefit both payers and patients.
7 Must-Have Features of High-Performing Clinical Registry Software
Choosing the right clinical registry software is not just a procurement decision; it’s an investment in a strategic partner that can transform your data management capabilities. The best platforms are more than just databases; they are powerful engines for research, quality improvement, and patient care. We’ve identified seven critical features that define a high-performing platform. For more on essential requirements, see our article on Four Key Patient Registry Software Requirements.
1. Robust Security and Compliance
Trust and patient privacy are the non-negotiable foundation of any clinical registry. Top-tier software must not only comply with regulations like HIPAA in the U.S. and GDPR in Europe but also hold independent certifications like SOC2 Type II to verify its security controls. Key features include end-to-end data encryption (both in transit and at rest), granular role-based access controls, and complete audit trails that log every action. A true commitment to security is demonstrated through a “Privacy by Design” approach, where privacy considerations are embedded into the system’s architecture from the outset, not added as an afterthought. For more, see our guide on Preserving Patient Data Privacy and Security.
2. Seamless Interoperability and Data Integration
A registry’s value is directly tied to its ability to create a single, unified view of the patient journey. Effective software must break down data silos by unifying information from scattered sources like Electronic Health Records (EHRs), laboratory information systems (LIMS), imaging archives (PACS), and patient wearables. This requires strong, pre-built EHR integration with major systems (like Epic and Cerner), robust API management, and sophisticated data harmonization tools to map disparate data formats to a common standard. Adherence to modern interoperability standards like FHIR (Fast Healthcare Interoperability Resources) and HL7 is essential for ensuring smooth, automated data exchange and reducing the significant IT burden of manual integration.
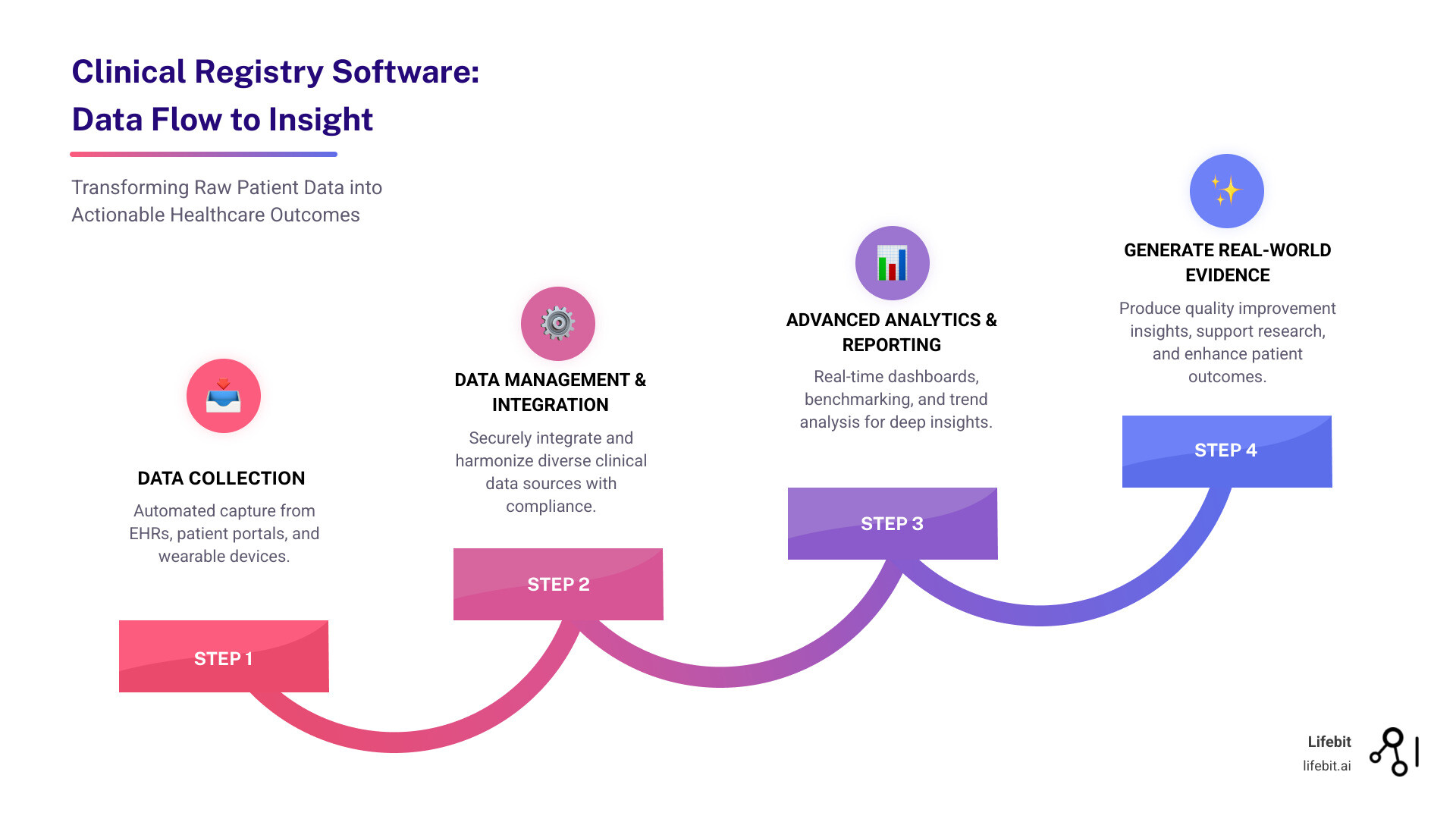
3. Advanced Analytics and Reporting
Great software turns raw data into actionable, evidence-based insights. Look for platforms that offer more than static reports. Key features include real-time dashboards for at-a-glance monitoring, dynamic benchmarking tools for quality improvement, and longitudinal trend analysis. Increasingly, AI and machine learning capabilities are critical differentiators. These enable powerful features like AI-powered cohort building (e.g., identifying patients for a trial based on criteria buried in unstructured clinical notes), semantic navigation of complex datasets, and automating the labor-intensive process of data abstraction to generate powerful real-world evidence faster than ever before.
4. Scalability and Flexibility
Research questions and data volumes evolve. A platform must adapt without requiring a complete overhaul. Key features include customizable workflows and data forms that can be modified without extensive coding, a modular design that allows you to add new functionalities as needed, and a cloud-based infrastructure. A cloud-native architecture is crucial for handling large datasets—including genomic, proteomic, and other multi-omic data—and provides the elastic scalability to manage growing data loads without large upfront investments in local IT hardware.
5. Comprehensive Data Management Tools
The credibility of your research depends on the quality and integrity of your data. Reliable insights require accurate, clean, and complete data. Essential tools include automated data validation rules at the point of entry, scheduled quality checks to flag inconsistencies or missing values, and integrated data cleaning features. For research-grade registries, version control with features like a “database lock” is critical. This allows youto freeze a dataset at a specific point in time for analysis or regulatory submission, ensuring data integrity, reproducibility, and traceability.
6. Patient Engagement and ePROs
Involving patients directly in the data collection process improves data completeness, captures the patient voice, and centers care around their lived experience. Modern registry software should include a secure patient portal where individuals can view their data and learn about research findings. Tools for collecting electronic patient-reported outcomes (ePROs) via surveys on web or mobile devices are essential. These must be supported by robust consent management workflows and secure patient communication features. This transforms a registry from a passive data repository into an active, patient-driven research community.
7. Support for Clinical Trial Matching
A modern registry can dramatically accelerate clinical research by bridging the gap between observational data and interventional trials. Advanced software should include features for cohort identification, allowing researchers to query the registry to find eligible patients for upcoming trials in minutes, not months. The software should also support the entire patient recruitment workflow and enable protocol feasibility analysis, using existing real-world data to model the potential success of a study design before it even begins. Learn more about Enhancing Clinical Trial Matching with Lifebit Patient Management.
Overcoming Challenges and Choosing the Right Platform
Implementing and maintaining a successful clinical registry software platform involves navigating a complex landscape of technical, regulatory, and organizational challenges. Understanding these issues upfront and planning for them is key to ensuring the long-term success and impact of your registry. For more detail, see our article on Challenges Facing Patient Registries in the US.
Common Implementation and Maintenance Challenges
Successfully launching a registry is only the beginning. The most significant challenges often emerge during long-term operation:
- Data Quality and Completeness: The principle of “garbage in, garbage out” is paramount. Inaccurate or incomplete data, often stemming from manual entry errors, varying documentation practices, and missing records, can severely undermine the validity of any analysis. Maintaining high-quality data requires continuous effort and robust software tools.
- Integrating Disparate Systems: A major technical pain point is integrating the registry with existing health IT infrastructure, especially legacy EHRs, lab platforms, and billing systems that may lack modern APIs. This often requires significant custom development and deep technical expertise.
- Ensuring Patient Privacy and Security: Beyond simply meeting the baseline requirements of regulations like HIPAA and GDPR, organizations must protect against data breaches, which can cause irreparable reputational damage and erode patient trust. This requires ongoing vigilance and a proactive security posture.
- Long-Term Financial Sustainability: Many registries are initiated with grant funding, which is often time-limited. Securing a sustainable financial model to cover long-term operational costs for staffing, software maintenance, and infrastructure is a critical, non-technical challenge.
- Evolving Regulatory Requirements: The regulatory landscape for data privacy, security, and the use of real-world evidence is constantly changing. Registries must have the agility to adapt their processes and software to remain compliant.
- Maintaining Stakeholder Buy-In: A registry’s success depends on the active participation of numerous stakeholders, including clinicians, administrators, IT staff, and patients. If data entry is burdensome for clinicians or if the registry fails to deliver tangible value back to the participants, engagement will wane over time.
How to Select the Right Clinical Registry Software for Your Needs
Choosing the right software partner is one of the most critical decisions you will make. A thoughtful, structured evaluation process will help you find a solution that aligns with your long-term vision:
- Define your research goals and scope to create a clear list of “must-have” versus “nice-to-have” features.
- Assess your technical requirements, including specific EHR integration needs, expected data volume, and the skill set of your internal IT team.
- Evaluate vendor expertise and support, looking for a partner with a proven track record in your specific area of healthcare and a responsive, knowledgeable support team.
- Request live, customized demos that reflect your specific workflows and use cases, not just a generic presentation.
- Check for certification and compliance with all relevant standards, including HIPAA, GDPR, and SOC2 Type II. Ask for documentation.
- Consider total cost of ownership (TCO), which includes not only the initial license fee but also costs for implementation, maintenance, training, and potential customization.
- Prioritize interoperability and customization to ensure the platform can connect with your existing systems and adapt as your program grows.
- Review the vendor’s product roadmap to ensure their future development plans align with emerging trends like AI and federated learning, effectively future-proofing your investment.
This strategic approach helps you find a platform that becomes a true partner in advancing research and improving patient care.
The Future of Patient Registries: Trends and Innovations
The world of patient registries is rapidly evolving, driven by new technologies and a growing demand for real-world evidence. The future of clinical registry software promises more powerful capabilities and a greater impact on healthcare.
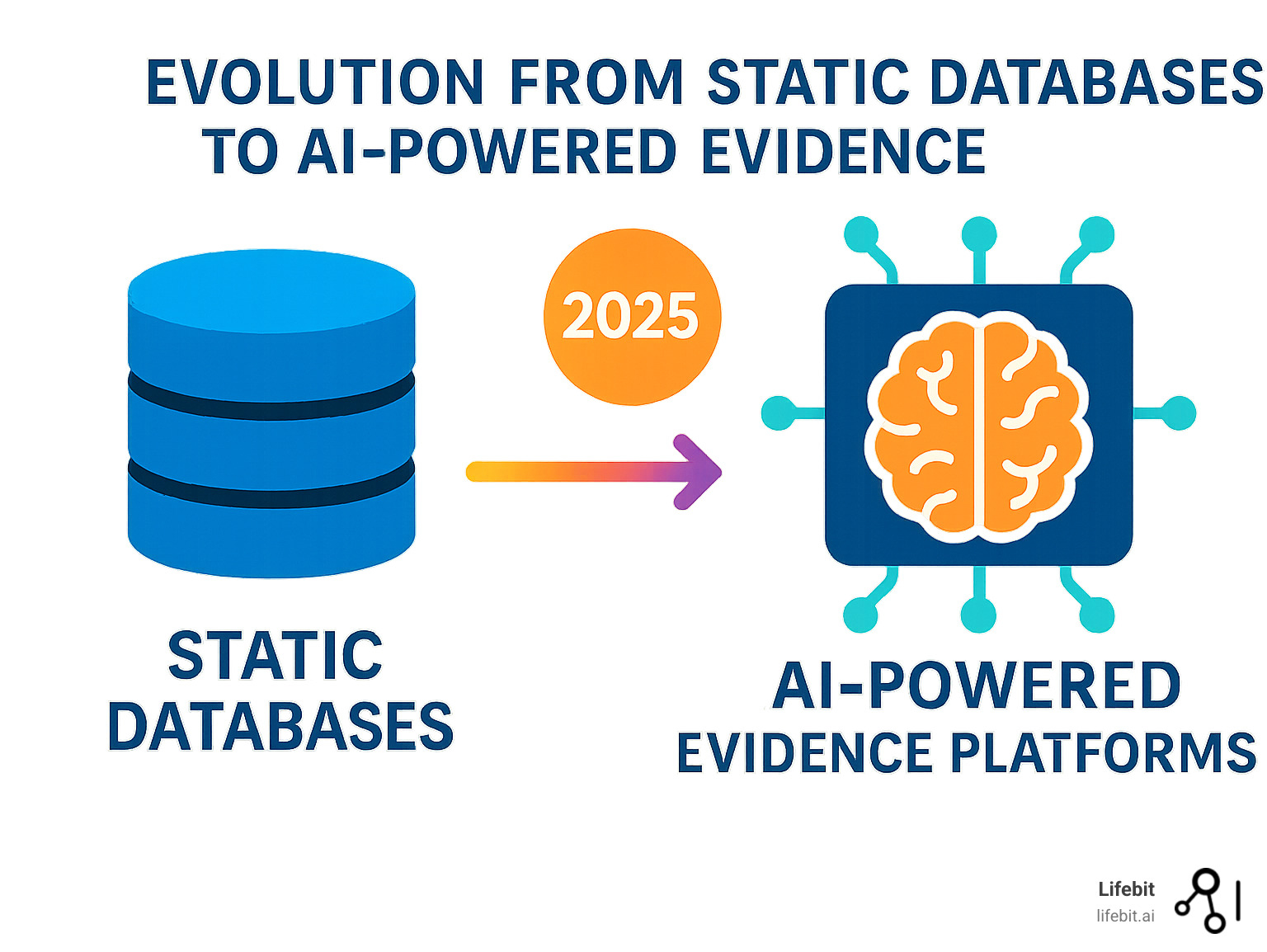
The Rise of AI and Real-World Evidence (RWE)
The growth of the Global Patient Registry Platform Market is tied to the increasing importance of AI and Real-World Evidence (RWE). AI and machine learning are changing registries from historical records into predictive tools. They can identify complex patterns, predict disease progression, and automate data abstraction. This helps providers anticipate patient needs and intervene proactively. Furthermore, regulatory bodies are increasingly accepting RWE from registries to support drug approvals and safety monitoring, boosting the industry’s value.
Federated Networks for Secure Collaboration
Federated networks are a groundbreaking trend enabling large-scale research without compromising patient privacy. With federated learning, sensitive patient data never leaves its original location. Analytical models are trained across distributed datasets, allowing researchers to analyze data from multiple sites or countries securely.
This approach fosters global research collaboration by eliminating the need to centralize data, which lowers security risks and simplifies compliance. Secure environments like Trusted Research Environments (TREs) provide controlled access for analysis, ensuring data use is auditable and secure. This strategy open ups the full potential of patient registries for advancing precision medicine while upholding privacy.
Conclusion
The evolution of clinical registry software into intelligent, AI-powered platforms is a major healthcare advance. These tools turn complex data into actionable insights that drive better care, innovative research, and improved patient outcomes.
The impact is significant, with registries contributing to a 24% decrease in postoperative mortality and a 30% reduction in opioid prescribing in key studies. From tracking rare diseases to monitoring medical devices and enabling global research via federated networks, these platforms are the backbone of evidence-based medicine.
Looking forward, the integration of advanced analytics, AI, and federated learning will open up deeper insights while maintaining patient privacy. The market’s projected growth to $3.12 billion by 2029 reflects a commitment to building a healthcare system that continuously learns and improves.
At Lifebit, we are proud to help organizations harness their data’s full potential with our federated AI platform. We are dedicated to making precision medicine the standard of care.
Ready to transform your data into impact? Visit https://lifebit.ai/platform/ to learn how our platform can help.


