Choosing Your Champion: The Best Clinical Trial Patient Recruitment Solutions

Clinical trial patient recruitment companies: 2025’s Best
Why Clinical Trial Patient Recruitment is Make-or-Break for Drug Development
Clinical trial patient recruitment companies are specialized organizations that help find and enroll eligible patients for medical studies. Using strategies from digital marketing to community partnerships, they connect patients with potentially life-saving treatments.
Key types of recruitment companies include:
- Digital-first agencies using social media, Google ads, and patient databases
- Full-service providers offering end-to-end recruitment and retention support
- Technology platforms enabling decentralized trials and virtual participation
- Community specialists partnering with patient advocacy groups and physician networks
The stakes couldn’t be higher. 85% of clinical trials are delayed due to patient recruitment challenges, costing an additional $1 million for every month of delay. With recruitment making up 40% of trial expenditures, choosing the right partner is critical.
The numbers tell a sobering story: 80% of studies fail to meet enrollment deadlines, and 35% fail to meet their targets entirely. Yet 75% of patients surveyed would have enrolled if they had known a trial was available.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve built a federated genomics platform that transforms how organizations access and analyze biomedical data for research. Through our work with pharmaceutical organizations and public sector institutions, I’ve seen how clinical trial patient recruitment companies can accelerate or derail drug findy timelines.
Basic clinical trial patient recruitment companies vocab:
- clinical trial recruitment strategies
- digital clinical trial recruitment
- clinical trial recruitment digital results case study
- Clinical trial
The High Stakes: Why Patient Recruitment is a Critical Bottleneck
After years of development and millions in investment, a promising treatment reaches the clinical trial phase, only to hit a wall: finding the right patients. This is a familiar story for pharmaceutical companies, as 85% of clinical trials struggle to recruit enough patients. These challenges create massive bottlenecks that can make or break entire research programs.
The problem is critical: 40% of all trial expenditures go toward recruitment efforts. Considering the average cost to bring a new drug to market exceeds $2.6 billion, those recruitment dollars add up fast. The competition is also fierce, with thousands of trials running simultaneously, all competing for a limited pool of eligible participants, particularly in crowded therapeutic areas like oncology and immunology.
The Staggering Cost of Delays
When recruitment falls behind, the financial impact is severe. Every month of delay can cost an additional $1 million in direct operational expenses. However, the true cost is far greater. Delays erode the effective patent life of a new drug, shrinking the window of market exclusivity. A six-month delay not only adds $6 million in direct costs but can also represent hundreds of millions in lost revenue if it allows a competitor to reach the market first.
Phase III studies face particularly brutal challenges, with recruitment difficulties causing roughly 30% of all delays. This means a life-saving treatment sits on the shelf while costs pile up. This has a cascading effect on a company’s portfolio. A six-month recruitment delay doesn’t just add expenses; it pushes back market entry, delays revenue streams, and fundamentally alters return on investment calculations. This can make investors nervous and jeopardize funding for other promising compounds in the drug development pipeline. Meanwhile, patients who could benefit from the treatment continue waiting, creating a vicious cycle where delayed trials consume resources that could have funded new research.
Primary Challenges in Finding Participants
Finding trial participants is difficult due to a perfect storm of interconnected challenges that even the most experienced sponsors struggle to overcome without specialized help.
Patient awareness is a core problem. A staggering 85% of patients are unaware that clinical trials exist or are an option for them. This is not due to a lack of interest—three-quarters of people would be willing to participate if they simply knew trials were available. The gap exists because there is no single, centralized, patient-friendly database for all trials. Patients are often left to navigate complex registries like ClinicalTrials.gov, which are designed for researchers, not the general public.
Physician communication creates another gap. Only 32% of patients report that their doctors clearly explained trial opportunities. This isn’t necessarily due to negligence. Many physicians lack the time during brief appointments to discuss complex research options, are unaware of trials happening outside their own institution, or may even have liability concerns about recommending an investigational treatment.
The complexity of modern protocols adds another layer of difficulty. The era of precision medicine means eligibility criteria are no longer as simple as “adults with type 2 diabetes.” Today’s protocols are intricate, requiring specific biomarkers, genetic profiles, and detailed medical histories. For example, a trial for a new lung cancer drug might require not just a specific cancer type (non-small cell lung cancer) but also a particular genetic mutation (e.g., KRAS G12C), no prior treatment with a similar drug, and specific organ function levels. This can shrink the pool of eligible participants from thousands of patients to a mere handful at any given clinical site.
Geographic barriers create practical roadblocks. The traditional model requires patients to travel to a major academic medical center, which may be hours away. For patients who are already ill, this burden is immense. It involves not just travel costs and time off work, but also arranging childcare, finding accommodation for multi-day visits, and the sheer physical exhaustion of travel. These logistical hurdles are a primary reason patients decline to participate or drop out of studies.
Health literacy and trust issues add emotional complexity. Clinical trial information can be dense and filled with medical jargon, making it overwhelming for patients and their families. Furthermore, historical abuses in medical research, such as the Tuskegee Syphilis Study, have created a deep-seated and justified skepticism, particularly within minority and underserved communities. Building trust takes time, cultural competency, and transparent communication—a luxury that aggressive recruitment timelines rarely allow. These combined challenges explain why specialized support from clinical trial patient recruitment companies has become essential.
A Guide to Leading Approaches in Clinical Trial Patient Recruitment

The world of clinical trial patient recruitment companies has transformed dramatically. What was once a process of posting flyers in doctors’ offices has evolved into a sophisticated ecosystem of digital platforms, AI-powered matching, and patient-centered approaches. Today’s recruitment strategies are like operating a high-tech fishing fleet with sonar, GPS, and multiple specialized nets, all designed to find the right fish in a vast ocean.
The shift is driven by necessity. With 80% of internet users seeking healthcare information online, recruitment companies must meet patients where they are: online, in their communities, and through their trusted healthcare providers.
Digital-First Recruitment Strategies
Digital recruitment is the backbone of modern patient enrollment. When someone receives a diagnosis, their first instinct is often to search online, and smart recruitment companies are there to meet them.
Digital advertising has evolved beyond simple banner ads. Leading firms now use thousands of digital channels to reach potential participants. This includes Google Ads targeting keywords related to conditions and treatments, and highly specific social media campaigns on platforms like Facebook, Instagram, and TikTok. The precision is remarkable, allowing campaigns to target individuals based on demographics, interests, and online behavior. Advanced firms continuously A/B test ad copy, images, and calls-to-action to optimize performance. They also focus heavily on Search Engine Optimization (SEO), ensuring that their patient-friendly trial landing pages rank highly in search results for relevant terms.
Patient databases are another powerful tool. Some companies have built proprietary communities of hundreds of thousands, or even millions, of patients who have already expressed interest in research. These are not cold leads; they are engaged individuals actively seeking to contribute to medical progress, who can be quickly matched to new trials.
Direct-to-patient outreach cuts through traditional gatekeepers. Custom-built patient portals and websites receive millions of views annually, allowing patients to search for trials, read about studies in plain language, and complete pre-screening questionnaires directly. This empowers patients and delivers more qualified referrals to sites.
Comprehensive Recruitment Support
Some clinical trial patient recruitment companies act as strategic partners, recognizing that success is about supporting the entire trial ecosystem—patients, sites, and sponsors—throughout the journey.
Strategic planning and patient journey mapping are foundational. Firms with decades of experience provide deep institutional knowledge. They begin by mapping the entire patient experience, from initial awareness to post-trial follow-up, to identify potential barriers and communication touchpoints. This informs a bespoke recruitment strategy tailored to the specific therapeutic area and patient population.
Site support and feasibility is increasingly important. Many providers offer pre-screening services that can reduce the administrative burden on clinical sites by up to 75%. A centralized contact center, staffed by clinical professionals, can handle initial screening calls, answer patient questions, and schedule appointments, passing only highly qualified and engaged candidates to site coordinators. Some firms also assist with site feasibility, identifying high-performing research sites and helping them get activated more quickly.
Patient retention services address the crucial task of keeping patients engaged. This is no longer just about sending appointment reminders. It includes providing transportation and lodging assistance, managing stipend payments, and offering technology solutions that make participation easier. Flexible service models allow sponsors to select a-la-carte services or a fully integrated, end-to-end solution.
Technology-Driven and Decentralized Solutions
The rise of decentralized clinical trials (DCTs) is a game-changer, especially for patients in rural areas or with mobility limitations.
Decentralized and virtual trials are now mainstream. Specialized companies use telehealth platforms, mobile technology, and a network of home health professionals to bring trials directly to patients’ homes. This can involve remote consenting, video consultations with investigators, and data collection via mobile apps. Entire technology platforms have been built around virtual trial execution.
Mobile apps and wearable technology are revolutionizing data collection. Custom apps for trials can handle patient-reported outcomes, medication diaries, and communication. Wearable sensors like continuous glucose monitors (CGMs), ECG patches, and activity trackers can passively collect objective, real-world data, reducing the burden on patients and providing richer insights for researchers.
Patient enrollment platforms provide sponsors with rich data analytics to drive faster enrollment. These platforms integrate with various recruitment channels and provide real-time dashboards to track key performance indicators (KPIs). Comprehensive software designed for modern, patient-centric trials offers user-friendly interfaces for sites, sponsors, and patients, unifying the trial experience.
Community and Physician Engagement
Despite technological advances, the human element remains irreplaceable. Trust and community connections are often decisive factors for patients.
Patient advocacy groups represent motivated and well-informed communities. Recruitment firms actively partner with these organizations. For example, in a cystic fibrosis trial, a firm might partner with the Cystic Fibrosis Foundation to host educational webinars, co-develop trusted materials, and access the foundation’s patient registry to identify individuals who have consented to be contacted about research.
Community partnerships create authentic connections. This involves working with local clinics, pharmacies, and community leaders to raise trial awareness. Some firms have built large online communities supporting patients with chronic and rare diseases, offering recruitment services that feel more like peer support than marketing.
Physician networks are crucial. Specialized firms focus on building and maintaining relationships with referring physicians. They provide tools, such as simple referral apps and dedicated portals, that make it easy for busy clinicians to identify potential candidates and track their progress, often providing compensation for the time spent on screening and referral activities. Even traditional methods like direct mail have a place, effectively reaching specific local populations who may be less digitally connected.
How to Select the Right Partner: A Checklist for Sponsors
Selecting the right clinical trial patient recruitment companies is a critical decision that can define the success of a study. The best partnerships are not just about who can deliver the most leads, but about finding a company that understands your trial’s unique challenges, integrates seamlessly with your clinical sites, and shares your commitment to patients. A great partner accelerates your timeline and reduces costs, while the wrong choice can lead to significant delays and wasted resources.
Key Factors for Choosing Clinical Trial Patient Recruitment Solutions
When evaluating potential partners, several factors are paramount.
Therapeutic area experience should top the list. You want a company that has a proven track record in your specific disease area. They will understand the patient journey, the competitive landscape, and which messages resonate. Always ask for detailed, relevant case studies with verifiable results.
Global reach versus local expertise is another critical consideration. Large, international trials require a partner with the infrastructure and knowledge to navigate different healthcare systems, cultures, and regulatory environments. For studies focused on specific regions or rare diseases, however, a partner with deep local community connections and physician networks may be more valuable.
The technology platform can make or break the patient and site experience. Look for partners with robust, user-friendly pre-screening tools, patient engagement platforms, and data management capabilities that can integrate with your existing CRO or sponsor systems (e.g., CTMS, EDC).
Cost models vary dramatically. Common models include pay-per-lead, pay-per-enrolled-patient, and monthly retainer fees. A pay-per-enrolled model aligns the partner’s incentives with yours but can be more expensive. Retainers provide budget predictability but may not guarantee results. Understand the pros and cons of each and find a model that aligns with your budget and risk tolerance.
Scalability and flexibility are essential. Your partner must be able to pivot quickly, scaling efforts up or down in response to real-time enrollment data. This includes the ability to launch “rescue” campaigns for a struggling trial or, conversely, to pause campaigns when a site is overwhelmed.
Measuring Success: The Metrics That Matter
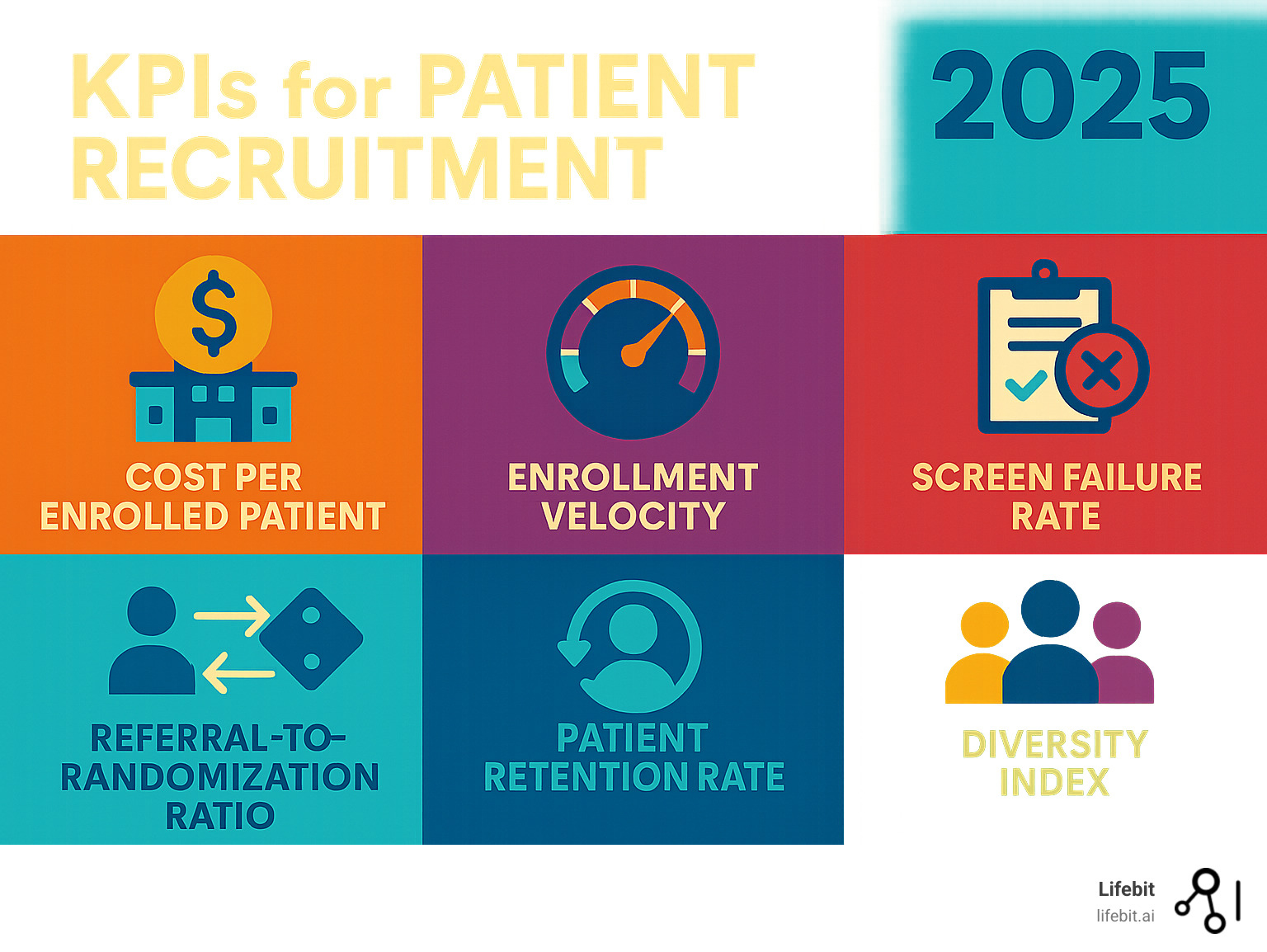
You can’t manage what you don’t measure. Success isn’t just about lead volume; it’s about enrollment quality and efficiency.
Cost per enrolled patient (CPEP) reveals the true financial efficiency of your recruitment spend. It’s calculated by dividing the total recruitment cost by the number of actual randomized patients, not just leads.
Enrollment velocity (patients per site per month) helps predict whether you’ll meet deadlines. This metric should be tracked in real-time to identify and address underperforming sites or strategies quickly.
Screen failure rate is a crucial quality metric. A high rate (>80-90% in many studies) indicates that advertising is poorly targeted or the pre-screening process is ineffective, which wastes valuable site time and resources.
The referral-to-randomization ratio reveals the health of your recruitment funnel. Tracking conversion rates at each step (e.g., lead > pre-screened > consented > randomized) helps pinpoint exactly where in the process patients are dropping off.
Patient retention rate reflects long-term engagement. Quality recruitment leads to better-informed and motivated patients who are more likely to complete a study. Top-tier partners contribute to retention rates of over 95%.
Participant diversity is essential for health equity and for producing generalizable data. Your partner must have a concrete plan and specific metrics for reaching underrepresented populations, tracking enrollment against the epidemiology of the disease.
Ensuring Diversity and Ethical Compliance in Your Recruitment Strategy
Diversity and ethics are not afterthoughts; they are foundational to a successful recruitment strategy.
Diversity and inclusion plans must be built into the initial strategy. The best clinical trial patient recruitment companies will help with protocol design feedback, site selection in diverse communities, and the development of culturally and linguistically appropriate outreach materials.
Navigating the IRB/EC submission process is a key service. Recruitment partners should be experts in crafting patient-facing materials (ads, websites, screeners) that are clear, non-coercive, and compliant with regulatory standards. They manage the submission process, saving sponsors and sites significant administrative time.
Ethical considerations are non-negotiable. All materials and interactions must prioritize patient wellbeing, providing a balanced view of the potential risks and benefits of participation without pressure or unrealistic promises.
Data privacy and security deserve special attention. Your partner must have robust, documented systems for protecting sensitive health information and complying with regulations like HIPAA in the US and GDPR in Europe. The right partner becomes an extension of your team, sharing your commitment to scientific rigor and patient care.
The Future of Recruitment: AI, Big Data, and Patient-Centricity
The world of patient recruitment is changing right before our eyes. We’re witnessing a shift toward AI-powered platforms, sophisticated data analytics, and truly patient-centered approaches that are reshaping how clinical trial patient recruitment companies operate. The future isn’t just about finding more patients faster—it’s about finding the right patients with precision while making the entire experience smoother for everyone involved.
These technological advances are directly addressing the core challenges of trial delays and costs. AI and big data are starting to chip away at these problems in meaningful ways, turning the vast amounts of biomedical data from a burden into a powerful asset that accelerates discovery.
The Rise of Data-Driven Cohort Finding
The days of casting a wide net and hoping for the best are rapidly ending. Today’s most innovative clinical trial patient recruitment companies are using real-world data (RWD) and artificial intelligence to pinpoint eligible patients with laser-like precision.
AI-powered platforms are revolutionizing how we identify potential participants. These systems can analyze structured data (like diagnosis codes and lab values) and unstructured data from electronic health records (EHRs), patient databases, and even genomic information to create highly targeted lists of candidates. Instead of broad digital advertising, AI helps focus efforts on individuals who are genuinely likely to qualify.
Leveraging unstructured data with NLP is a key innovation. A significant portion of a patient’s story is locked in unstructured clinical notes. AI using Natural Language Processing (NLP) can read and interpret these notes to identify nuanced criteria—like disease stage or specific symptoms—that are not captured in structured fields. This dramatically improves the accuracy of EHR-based matching.
Federated learning for privacy preservation is another critical advance. For precision medicine trials, it’s often necessary to analyze data from multiple hospitals or research centers. Federated learning allows AI models to be trained on this distributed data without the sensitive patient data ever leaving the institution’s firewall. This enables powerful, large-scale analysis while maintaining the highest standards of patient privacy and data security.
Predictive analytics takes this even further. Rather than just finding patients, these systems can forecast enrollment rates, predict which recruitment strategies will be most effective for specific populations, and even identify potential bottlenecks before they derail a study. It’s like having a crystal ball for clinical trial planning.
How Technology Improves Patient Retention and Site Efficiency
Finding patients is only half the battle—keeping them engaged is equally critical. Technology is making remarkable strides in both improving the patient experience and reducing the administrative burden on clinical sites.
Patient engagement platforms are becoming the central hub for a participant’s trial journey. Modern mobile-first portals give participants a single, user-friendly place to manage their experience. They can access study documents, receive personalized updates, communicate with the research team, and complete surveys. This convenience and transparency significantly improve satisfaction and retention.
eConsent processes are streamlining one of the most critical steps. Interactive digital consent forms, which can include videos and embedded definitions, are easier for patients to understand than dense paper documents. They allow for better documentation of understanding and can be completed remotely, reducing site burden and patient travel.
Automating patient support reduces friction. Technology platforms can now automate the entire patient reimbursement process, allowing participants to upload receipts for travel and receive payments quickly via digital wallets or direct deposit. This removes a common source of frustration and financial strain.
For clinical sites, centralized portals are game-changers. These systems can handle medical record retrieval, biospecimen tracking, and outcome verification from a single interface. By automating administrative tasks, these platforms can speed up site follow-ups by 75% and allow sites to reach four times as many patients.
Real-time data integration from multiple sources—EHRs, wearables, patient-reported outcomes—creates comprehensive dashboards. These give research teams immediate insights into recruitment progress, patient engagement, and data quality, enabling quick course corrections and more informed decision-making.
At Lifebit, we’re seeing how federated AI platforms can securely access and analyze global biomedical data while maintaining privacy and compliance. This approach enables researchers to identify eligible patients across multiple data sources without compromising sensitive information—a crucial capability as trials become more complex and patient populations more specific.
The future of clinical trial recruitment isn’t just about better technology—it’s about creating a more human, accessible, and efficient experience for everyone. As these innovations mature, we expect to see dramatic improvements in enrollment success rates and patient satisfaction.
Conclusion
The world of clinical research is changing before our eyes, and clinical trial patient recruitment companies are at the heart of this revolution. What once seemed like an impossible challenge—finding the right patients for the right trials—is becoming more achievable through innovation, collaboration, and a genuine commitment to putting patients first.
We’ve seen how the stakes are incredibly high. With 85% of trials facing delays and costs spiraling into millions per month of delay, the pressure is real. But we’ve also witnessed remarkable solutions emerging from this challenge. Digital-first strategies are reaching patients where they already spend their time. Comprehensive support services are taking the burden off overwhelmed research sites. Technology-driven platforms are making participation possible for patients who could never travel to traditional sites.
The shift toward data-driven, tech-enabled recruitment isn’t just about efficiency—it’s about equity and access. When we can identify eligible patients faster through AI-powered platforms, when we can bring trials to patients through decentralized models, and when we can ensure diverse representation through thoughtful outreach strategies, we’re not just speeding up drug development. We’re making it more inclusive and more human.
Choosing the right strategic partner has never been more critical. It’s about finding a company that doesn’t just promise numbers, but delivers quality referrals, maintains ethical standards, and truly understands your therapeutic area. The best clinical trial patient recruitment companies become extensions of your team, sharing your commitment to bringing life-changing treatments to patients who need them.
The future is incredibly promising. AI and big data are revolutionizing how we identify patient cohorts. Federated platforms are enabling secure access to global datasets while protecting patient privacy. Real-time analytics are helping us predict and prevent recruitment bottlenecks before they happen.
At Lifebit, our federated AI platform enables secure access to global biomedical data, accelerating patient identification for complex trials while ensuring privacy and compliance. We’re helping researchers harness the power of diverse datasets to find the right patients faster, ultimately bringing life-saving therapies to those who need them most.
The path from laboratory findy to patient treatment doesn’t have to be as long or as difficult as it once was. With the right partner and the right technology, we can make clinical trials more accessible, more efficient, and more successful than ever before.
Find how to accelerate research with a next-generation data platform


