Beyond the Buzzwords: What Are Real-World Data and Evidence?

Real World Data vs Real World Evidence: Crucial 101
Why Understanding Real-World Data vs Real-World Evidence Matters for Healthcare Leaders
Real world data vs real world evidence are two terms that sound similar but have distinct meanings in healthcare research. Understanding this difference is critical for making informed decisions about drug approvals, safety monitoring, and healthcare policy. Here’s the key distinction:
| Real-World Data (RWD) | Real-World Evidence (RWE) |
|---|---|
| Raw health data collected outside clinical trials | Clinical insights derived from analyzing RWD |
| Examples: EHRs, claims, registries, wearables | Examples: Safety studies, effectiveness reports |
| What it is: The ingredients | What it is: The recipe outcome |
| Purpose: Data collection | Purpose: Decision-making evidence |
Traditional randomized controlled trials (RCTs), the gold standard since 1962, are expensive, slow, and often exclude patients who will use treatments in real life—such as pregnant women, the elderly with multiple conditions, and diverse populations. This creates an “efficacy-effectiveness gap”—the difference between a treatment’s performance in controlled trials versus everyday clinical practice. RWE helps bridge this gap by showing how treatments work for real patients in real-world settings.
The regulatory landscape is evolving. The FDA’s 21st Century Cures Act and RWE Framework have opened new pathways for using real-world evidence in drug approvals, while the EMA has developed comprehensive data quality frameworks. The urgent need for harmonization is clear, with at least six major organizations having published formal definitions of RWD.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. For over 15 years, I’ve helped organizations transform complex biomedical data into actionable insights using federated analytics, focusing on the secure analysis of real world data vs real world evidence for pharma and public sector clients globally.
What is Real-World Data (RWD)? The Raw Ingredients of Insight

Real-World Data (RWD) is health information collected outside the controlled environment of clinical trials. It’s the authentic data generated as patients live their daily lives and interact with healthcare systems. Unlike standardized clinical trials, RWD captures the real story of how diseases progress and treatments work in the wild.
This data reveals what happens when a 75-year-old with diabetes and heart disease takes a new medication, not just how it performed in a study of healthy 30-year-olds. It provides patient journey data from diagnosis through treatment, painting a complete picture of healthcare in action. When comparing real world data vs real world evidence, RWD is the raw material that researchers transform into insights. For more, see our guide on the real-world data definition.
Primary Sources of RWD and Their Characteristics
RWD’s value lies in its diversity, coming from every point of patient interaction with healthcare.
- Electronic Health Records (EHRs) are a rich source, containing digital patient charts. This includes structured data like diagnosis codes and lab values, and unstructured data like clinical notes and imaging reports. Extracting insights from unstructured text often requires advanced techniques like Natural Language Processing (NLP).
- Medical claims data, generated from insurance billing, uses standardized codes (e.g., ICD-10 for diagnoses, CPT for procedures) to track what healthcare services patients used and when. This structure facilitates large-scale analysis but lacks the clinical granularity of EHRs.
- Disease and product registries are focused data collections, such as the Surveillance, Epidemiology, and End Results (SEER) Program for cancer research or registries tracking patients with specific medical devices. They are goldmines for understanding specific conditions in depth.
- Patient-generated data is a rapidly growing category. It includes data from wearables (e.g., heart rate, activity levels), but also Patient-Reported Outcomes (PROs) collected via apps and surveys, which capture crucial subjective information like pain levels, quality of life, and symptom severity.
- Genomic data, often sourced from large-scale biobanks like the UK Biobank or the All of Us Research Program, links genetic information with clinical data. This combination is essential for advancing personalized medicine and understanding the genetic basis of disease and treatment response.
You can see these sources in action in our collection of real-world data examples.
Strengths and Limitations of Different RWD Sources
Each RWD source has unique strengths and weaknesses. Understanding these is crucial for anyone working in the real world data vs real world evidence space.
EHRs offer great clinical depth but are often unstructured, incomplete, and difficult to integrate due to interoperability issues and quality problems like “copy-paste” notes. Claims data provides incredible longitudinal scale and is well-structured, but it’s designed for billing, not research. It lacks clinical detail (e.g., lab results) and can be misleading; for example, a claim for a dispensed drug doesn’t confirm the patient actually took it (adherence). Registries can be highly detailed but may suffer from selection bias, as their patient populations might not represent the general population. Spontaneous reporting systems for drug safety are vital but are estimated to capture only about 6% of actual adverse drug reactions, leaving a 94% blind spot.
RWD often comes in different formats and quality levels, making it a challenge to blend properly. We explore these complexities in our article on the challenges of using real-world data in research. Despite these problems, RWD offers an unprecedented opportunity to understand healthcare as it truly happens.
How is Real-World Evidence (RWE) Generated? The Path from Data to Findings
If RWD provides the puzzle pieces, Real-World Evidence (RWE) is the clear picture they form when assembled. RWE is the clinical evidence about a medical product’s usage, benefits, or risks that comes from analyzing Real-World Data. It’s the moment raw data becomes actionable knowledge that can guide treatment decisions and inform regulatory approvals.
The key difference in real world data vs real world evidence is transformation. RWD is potential waiting in a database; RWE actively answers critical questions like, “Does this drug work better for elderly patients?” This transformation requires scientific rigor and the right analytical approach.
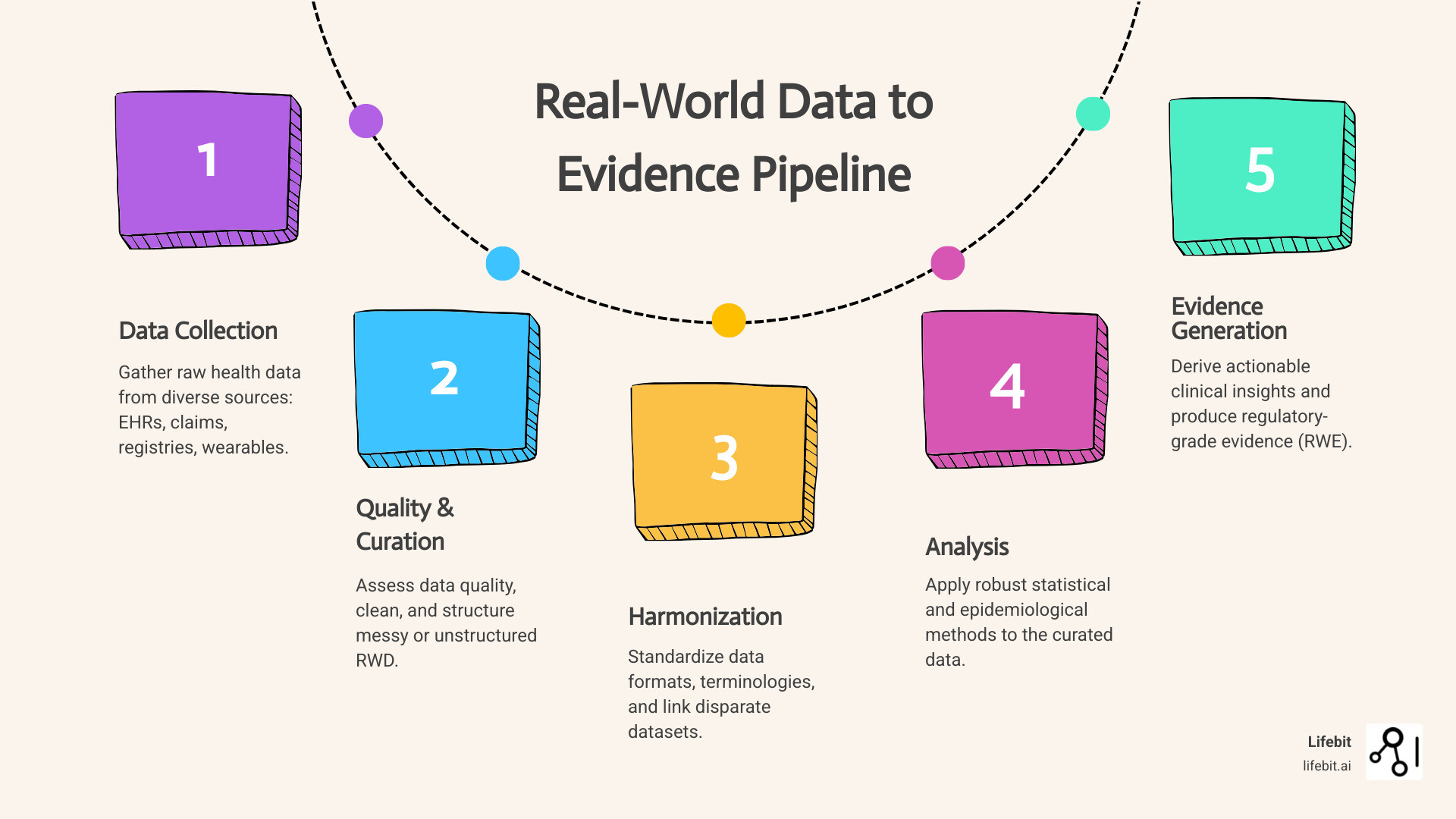
The Generation Pipeline: From Raw Data to Regulatory-Grade Evidence
Creating trustworthy RWE from messy RWD is a methodical, scientific process. The journey involves several critical steps to ensure the evidence can stand up to regulatory and clinical scrutiny.
- Data collection and curation: The process begins with a well-defined research question, which guides the identification of relevant RWD sources. Data is then curated, which involves cleaning, standardizing, and often mapping it to a Common Data Model (CDM) like OMOP to create a uniform structure.
- Data quality assessment: This crucial step evaluates if the data is “fit-for-purpose.” The EMA’s framework provides key dimensions: Reliability (is the data accurate?), Extensiveness (is the data complete?), Coherence (is it consistent across sources?), Timeliness (is it up-to-date?), and Relevance (does it contain the variables needed to answer the question?).
- Data harmonization: This technical process ensures different datasets “speak the same language,” so a measurement from one system means the same as a measurement from another. This is vital for multi-site studies. Our guide on Data Harmonization: Overcoming Challenges offers practical solutions.
- Study design and analysis: Researchers choose appropriate observational study designs (e.g., cohort studies, case-control studies) and apply advanced statistical methods to minimize bias and confounding. Techniques like propensity score matching or weighting are used to create comparable groups, mimicking the randomization of an RCT. More advanced approaches include target trial emulation.
- Interpretation and evidence generation: The final step transforms analytical results into clinically meaningful conclusions. This requires deep domain expertise to contextualize the findings and assess their impact on patient care or policy.
This entire process follows a systematic approach, detailed in our guide: A 7-step process for RWD.
Why the Distinction Between Real World Data vs Real World Evidence Matters
Understanding the real world data vs real world evidence distinction has practical implications for healthcare organizations.
- Input vs. Output: RWD is the raw ingredient; RWE is the finished dish, ready to inform decisions.
- Potential vs. Proof: RWD may suggest a correlation, but only RWE generated through rigorous analysis can provide proof of causation.
- Required Skills: Managing RWD requires data engineers, while generating RWE demands epidemiologists, biostatisticians, and clinical researchers.
- Regulatory Scrutiny: While RWD collection has privacy requirements, the generation of RWE faces intense scientific examination from bodies like the FDA and EMA.
The Critical Comparison: Real World Data vs Real World Evidence
Let’s place real world data vs real world evidence side by side to highlight the fundamental distinctions that can make or break a research project or regulatory submission.
| Feature | Real-World Data (RWD) | Real-World Evidence (RWE) |
|---|---|---|
| Definition | Routinely collected health-related data from outside traditional clinical trials. | Clinical evidence on a medical product’s usage, benefits, or risks, derived from analyzing RWD. |
| Format | Raw, unstructured or semi-structured datasets (e.g., EHR entries, claims codes). | Analyzed, interpreted findings presented as reports, conclusions, or statistical models. |
| Purpose | To capture real-world patient experiences and health outcomes as they naturally occur. | To generate actionable insights that inform healthcare decisions and regulatory approvals. |
| Example | A patient’s electronic health record detailing diagnoses, medications, and lab results. | A study report concluding a drug reduces hospitalization rates, based on analysis of claims data. |
| Key Challenge | Data quality, heterogeneity, missingness, and privacy concerns. | Methodological rigor, controlling for bias, generalizability, and interpretability. |
Think of it this way: RWD is all the individual ingredients in a grocery store. RWE is the finished meal created by a skilled chef, complete with a recipe explaining how it was made.
Key Differences in the Real World Data vs Real World Evidence Debate
The distinction becomes clear when looking at four key areas:
- Nature: RWD is raw and unprocessed, simply reflecting what happened. RWE is carefully analyzed and interpreted data that explains the why behind what happened.
- Purpose: RWD is collected and stored. RWE is generated to answer specific questions and solve real problems.
- State: RWD represents pure potential. RWE is that potential realized—actual insights ready for decision-making.
- Governance: For RWD, governance focuses on secure data access and privacy (HIPAA, GDPR). For RWE, it centers on ensuring scientific methods are sound, transparent, and reproducible.
These differences are critical. Access to millions of patient records is useless without the expertise and methodology to transform that RWD into reliable RWE. Conversely, sophisticated analytics cannot create trustworthy evidence from poor-quality data.
RWD and RWE in Practice: Applications and Regulatory Impact
The conversation around real world data vs real world evidence has shifted from academic to essential. These tools now guide decisions throughout a medicine’s lifecycle, from early research to long-term safety monitoring. We’ve moved from limited snapshots from clinical trials to a continuous movie of how treatments work in the real world.
Applications span the entire drug lifecycle:
- Drug discovery and development: RWD helps identify unmet medical needs, understand disease progression (natural history studies), and validate novel drug targets.
- Clinical trial optimization: RWD informs more realistic eligibility criteria and can be used to create external or synthetic control arms. This is particularly valuable in rare diseases or oncology, where recruiting a placebo or standard-of-care arm may be unethical or infeasible. The ADAPTABLE pragmatic trial is a key example of using RWD infrastructure for efficient trial execution.
- Regulatory submissions: RWE is increasingly used to support regulatory decisions. A landmark example is Pfizer’s Ibrance (palbociclib), which gained FDA approval for male breast cancer based on RWE from EHRs and claims data, extending its original indication without a new trial.
- Post-market surveillance: RWE has revolutionized safety monitoring by enabling active, continuous tracking of millions of patients to spot rare adverse events and understand long-term effectiveness in routine care.
How RWD/RWE Complements Randomized Controlled Trials (RCTs)
While RCTs remain the gold standard for proving efficacy under ideal conditions, those conditions rarely exist in real life. RCTs are costly, time-consuming, and use narrow populations with strict inclusion/exclusion criteria. This often excludes patients with multiple health conditions (comorbidities), those taking multiple medications (polypharmacy), or individuals from diverse demographic groups. This creates the “efficacy-effectiveness gap”—the difference between how a drug performs in a controlled trial versus in routine clinical practice.
RWE complements RCTs by providing broader generalizability. By analyzing data from heterogeneous, real-world populations, we can see how treatments work for the full spectrum of patients who will actually use them, including the elderly, pregnant women, and different ethnic groups often underrepresented in trials.
RWE also enables the assessment of long-term outcomes over decades, far beyond the typical duration of an RCT. This helps us understand if benefits last and what rare, long-term side effects might emerge. The RCT-DUPLICATE initiative has been groundbreaking, successfully emulating clinical trials with RWD and showing when real-world studies can reliably complement traditional trials.
The Role of Regulatory Bodies: FDA and EMA
Regulatory bodies like the FDA and EMA have embraced the potential of real world data vs real world evidence. The FDA’s 2016 21st Century Cures Act and subsequent Real-World Evidence Framework provide clear guidance on using RWE for regulatory decisions, with specific documents covering topics like “Assessing Registries to Support Regulatory Decision-Making” and “Use of Electronic Health Record Data in Clinical Investigations.” The agency has published numerous guidance documents on the topic, which you can explore on the FDA guidance on RWE page.
The European Medicines Agency (EMA), through the HMA-EMA Big Data Steering Group, has focused on its Data Quality Framework, which defines five dimensions for evaluating RWD: reliability, extensiveness, coherence, timeliness, and relevance. The EMA is also leading the DARWIN EU (Data Analysis and Real World Interrogation Network), which is establishing a federated network of data sources across the EU. Its goal is to provide timely and reliable evidence from routine healthcare data to support regulatory decision-making throughout a medicine’s lifecycle. Despite these advances, global harmonization of standards remains a key challenge and a major focus for international collaboration.
Ensuring Trust: Governance, Ethics, and the Future of RWE
The growing use of real world data vs real world evidence demands a commitment to ethical use, transparency, and quality. Without robust governance, RWE can be misleading. Think of it as needing safety measures and rules of the road before driving a powerful sports car.
Data quality frameworks, like the EMA’s five dimensions (reliability, extensiveness, coherence, timeliness, and relevance), provide a roadmap. Fitness-for-purpose assessment is crucial—ensuring the data is appropriate for the research question. Study transparency is also non-negotiable, with guidelines like STROBE and RECORD helping researchers report their methods clearly so others can evaluate and reproduce their findings. The CIOMS report on RWD/RWE offers further insights into these principles.
Critical Ethical and Governance Considerations
Using health data from millions of patients requires careful ethical navigation. Getting it right accelerates medical breakthroughs; getting it wrong erodes public trust.
Patient consent and data privacy are central concerns. Regulations like GDPR in Europe and HIPAA in the United States set strict legal requirements for handling personal health data. To meet these rules while enabling research, techniques like de-identification and pseudonymization are used to protect patient privacy.
Technical infrastructure has evolved to meet these challenges. Secure Data Environments (SDEs) create digital fortresses where sensitive data can be analyzed without leaving its secure location. You can learn more in our guide on what is a secure data environment.
Federated analytics platforms are the next step. Instead of moving data, these platforms bring the analysis to the data. At Lifebit, our federated approach allows researchers to analyze datasets across multiple locations without the data ever leaving its secure home. This is especially powerful for international research, where data protection laws vary. By keeping data in place, federated platforms help researchers steer complex regulations while maintaining the highest standards of data protection.
The path forward requires continued harmonization of ethics and governance frameworks to prevent regulatory patchwork from slowing down vital global research.
Frequently Asked Questions about RWD and RWE
As organizations steer the complexities of real world data vs real world evidence, several key questions consistently arise.
Can RWE replace RCTs for drug approval?
Not entirely, but RWE is a powerful complement. It is increasingly used for label expansions, studying rare diseases, and creating external control arms for trials where a placebo would be unethical. While RWE has supported some drug approvals, particularly in oncology, it does not yet replace pivotal RCTs for proving initial efficacy. However, its role in regulatory decision-making is growing as analytical methods improve.
What is the biggest challenge in using RWD?
Data quality and interoperability are the biggest problems. RWD is inherently messy, incomplete, and stored in different formats across various systems. This requires significant effort in data cleaning, standardization, and harmonization before it becomes research-ready. This challenge is especially acute in multi-national studies, where different coding standards and privacy regulations create a complex puzzle.
How does RWE help after a drug is on the market?
This is where RWE is most essential. In post-market surveillance, RWE acts as an early warning system for detecting rare adverse events that may not have appeared in smaller clinical trials. It allows for continuous monitoring of a drug’s long-term safety and effectiveness across a broad, diverse patient population in routine clinical practice. This helps us understand how a drug performs in the real world, informing label updates and clinical guidelines to ensure a treatment’s benefit-risk profile continues to evolve long after its initial approval.
Conclusion: From Data Points to Patient Impact
The journey from real world data vs real world evidence represents a major change in healthcare, turning scattered data points into life-changing insights.
- RWD is the raw material—the health records, claims, and wearable data that tell the unfiltered story of patient health.
- RWE is the finished masterpiece—the actionable insights generated when we apply rigorous scientific methods to that data.
This symbiotic relationship is changing medicine. We can now understand how treatments work for diverse populations often excluded from trials, spot safety signals faster, and make better-informed decisions. As regulatory frameworks like the FDA’s 21st Century Cures Act evolve, the ability to generate reliable RWE will become even more critical.
However, using this power requires sophisticated platforms that can manage data complexity while ensuring privacy and security. Organizations need systems that can harmonize data, apply advanced analytics, and enable secure collaboration.
Lifebit’s federated AI platform addresses these needs. With built-in capabilities for harmonization, AI/ML analytics, and federated governance, we help organizations transform raw biomedical data into actionable insights. Our platform enables real-time evidence generation and secure collaboration, allowing researchers to work with global datasets without compromising patient privacy.
The future of healthcare lies in turning everyday patient experiences into evidence that saves lives. Explore how federated technology is making this possible.

