What is Real World Data? A Comprehensive Guide to Data-Driven Insights
Real world data: The #1 Powerful Guide 2025
Why Real World Data is Changing Healthcare Decision-Making
Real world data is revolutionizing how we understand medicine effectiveness and patient outcomes beyond the controlled environment of traditional clinical trials.
Quick Answer: What is Real World Data?
- Definition: Data collected from routine healthcare delivery and patient experiences outside of controlled clinical trials
- Sources: Electronic health records, insurance claims, patient registries, wearable devices, and mobile health apps
- Purpose: Generate real-world evidence (RWE) to inform regulatory decisions, clinical practice, and healthcare policy
- Key Benefit: Provides insights into how treatments perform in diverse, real-world patient populations
The healthcare industry is shifting. While randomized controlled trials (RCTs) are essential for establishing safety and efficacy, they often fail to capture how treatments perform in the real world. Recognizing this, over 90% of life-science organizations currently use RWD in clinical development and decision-making.
Real world data bridges this gap by capturing information from diverse patient populations. The FDA’s creation of its Real-World Evidence Program Framework in 2018, following the 21st Century Cures Act, was a pivotal moment in legitimizing RWD as a cornerstone of modern evidence generation.
For pharmaceutical companies, regulators, and healthcare organizations, RWD offers unprecedented opportunities to understand treatment effectiveness, monitor safety, and optimize care. However, this data-rich environment also presents challenges around data quality, privacy, and analytical complexity.
As CEO and Co-founder of Lifebit, my 15+ years in computational biology and health-tech have focused on helping organizations harness real world data. Through secure, federated platforms, we enable collaboration without compromising patient privacy, changing drug findy and clinical decision-making.
Understanding the Fundamentals of Real World Data
Real world data is the continuous stream of health information generated from the everyday practice of medicine—every patient interaction, prescription, and test result.
Unlike the carefully orchestrated world of clinical trials, RWD captures the complexity of actual patient care. It shows how treatments perform when patients have multiple health conditions or struggle with adherence, providing a true picture of how healthcare really works.
What is Real World Data (RWD) and How Does It Differ from Clinical Trial Data?
Real world data is the digital footprint of healthcare as it happens. The FDA defines it as data about patient health and healthcare delivery collected during routine care. If clinical trials are like a choreographed performance, real world data is like watching people dance at a wedding—both have value but tell different stories.
Clinical trials, or Randomized Controlled Trials (RCTs), are designed to answer if a treatment works under perfect conditions. They recruit highly selected patients and control every possible variable. However, most patients don’t live in these ideal conditions. Real world data captures these realities, showing how treatments work for broader, more diverse populations.
| Feature | Real World Data (RWD) | Traditional Clinical Trial Data (RCT) |
|---|---|---|
| Patient Population | Broad, diverse, often heterogeneous, reflecting real-world patients and comorbidities | Highly selected, homogeneous, meeting strict criteria |
| Data Collection | Routinely collected from clinical practice, non-interventional | Prospectively collected with specific study protocols, interventional |
| Study Environment | Everyday clinical practice, uncontrolled | Controlled, structured, often academic settings |
| Cost | Generally lower, leveraging existing data | High, due to extensive setup, monitoring, and recruitment |
| Generalizability | High, reflects real-world effectiveness | Lower, may not fully apply to diverse patient populations |
| Primary Purpose | Understand real-world use, effectiveness, safety, and patient experience | Establish efficacy and safety for regulatory approval |
| Bias Control | More susceptible to confounding and selection bias, requires advanced analytical methods | High, due to randomization and strict protocols |
Combining both is key: RCTs tell us can a treatment work, while RWD tells us does it work for actual patients.
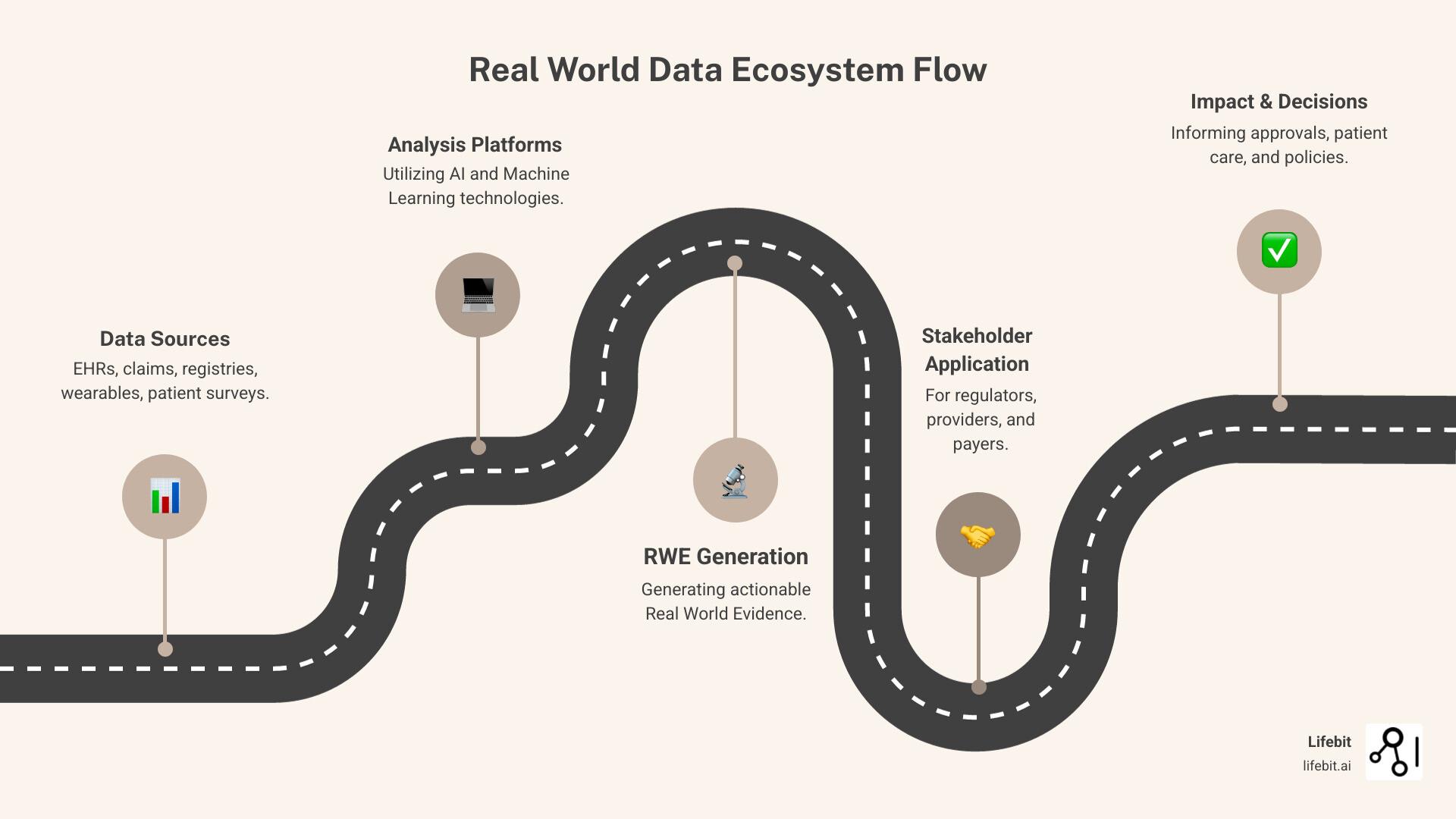
What are the Primary Sources of RWD?
The sources of real world data are as diverse as healthcare itself. Key sources include:
- Electronic Health Records (EHRs): The digital memory of healthcare, capturing diagnoses, prescriptions, lab results, and clinical notes.
- Claims and billing data: A massive database from insurance companies detailing which treatments patients receive and their costs.
- Patient-reported outcomes (PROs): Direct feedback from patients on their symptoms, quality of life, and treatment side effects.
- Disease registries: Detailed databases for specific conditions or treatments, especially valuable for rare diseases.
- Mobile devices and wearables: Continuous streams of health data from smartphones, smartwatches, and other personal health monitors.
- Genomic data: Information connecting a person’s DNA to their health outcomes, paving the way for personalized medicine.
Ensuring quality across these diverse sources is a key challenge, as highlighted by scientific research on data quality in clinical data networks.
From RWD to Real World Evidence (RWE): Generating Actionable Insights
Having real world data is only valuable if you can generate insights from it. This is where Real World Evidence (RWE) comes in. The FDA defines RWE as the clinical evidence derived from analyzing RWD.
Creating RWE requires careful statistical work and advanced techniques like causal inference to account for the observational nature of the data. The goal is to tease out meaningful relationships and avoid analytical traps.
The insights gained are remarkable. We can understand how treatments perform across different populations, identify safety signals missed in smaller trials, and track disease progression over time. This process turns raw data into actionable evidence that can improve patient care, inform regulatory decisions, and guide healthcare policy.
The Regulatory Landscape: RWD in Policy and Decision-Making
Over the past decade, real world data has moved from the sidelines to center stage in healthcare regulation, fundamentally changing how agencies evaluate medical treatments and devices. Regulators now recognize that to truly understand how treatments work for diverse populations, we need RWD.
This paradigm shift has been swift, with regulatory bodies worldwide actively embracing RWD for drug approvals, post-market surveillance, and medical device evaluation. This global harmonization is accelerating medical innovation and helping bring treatments to patients faster.

The 21st Century Cures Act and the FDA’s RWE Framework
The United States took a giant leap forward in 2016 with the 21st Century Cures Act. This landmark legislation mandated that the FDA establish a program to evaluate how real world evidence could support regulatory decisions.
In 2018, the FDA released its groundbreaking “Framework for FDA’s Real-World Evidence Program,” available on the FDA’s website. This framework officially opened the door for RWE to be used for supporting approval of new drug indications and satisfying post-approval study requirements.
While the FDA had long used RWD for post-market safety monitoring, the Cures Act pushed them to use RWE for demonstrating treatment effectiveness. The framework established clear guidelines on data quality, analytical methods, and study design, giving companies the confidence to invest in RWD initiatives with a clear regulatory pathway.
Global Harmonization: EMA, NICE, and Other International Frameworks
Regulatory agencies across the globe have been converging on the value of real world data. This has led to international standards and collaborative opportunities.
The European Medicines Agency (EMA) has focused on creating a “learning healthcare system,” emphasizing the role of high-quality data from sources like patient registries, as detailed in their discussion paper on patient registries.
In the UK, the National Institute for Health and Care Excellence (NICE) published its RWE Framework in 2022, connecting real world evidence directly to value assessment and healthcare planning. NICE uses RWE to determine if treatments provide good value for the healthcare system.
This global harmonization is creating unprecedented opportunities for multi-regional studies that provide comprehensive insights across different healthcare systems. Regulatory agencies in Canada, Australia, Japan, and other countries are all developing their own RWD frameworks, accelerating medical innovation and ensuring decisions are based on the most complete evidence possible.
Key Applications and Benefits of RWD and RWE
The transformative power of real world data touches every corner of healthcare, from early drug findy to bedside treatment decisions. It acts as a bridge between clinical research and everyday medical practice, showing what actually happens when real patients receive treatments.
Accelerating Life Sciences and Clinical Development
The pharmaceutical industry has acceptd real world data, with over 90% of life-science organizations using RWD in their clinical development programs. This accelerates the journey from lab to bedside in several ways:
- Smarter trial design: RWD helps researchers understand disease epidemiology and standard of care to design more efficient trials. By analyzing RWD, teams can optimize inclusion/exclusion criteria, estimate recruitment potential, and ensure the protocol reflects real-world clinical practice. The ADAPTABLE trial, for example, showed that EHR-enabled studies can be run at a fraction of the cost of traditional RCTs.
- Faster patient recruitment: Instead of manual searches, researchers can use RWD to quickly identify potentially eligible participants from large EHR networks and registries. This is especially powerful for rare disease research, where patients are few and geographically dispersed.
- Synthetic control arms (SCAs): Instead of recruiting a concurrent control group, researchers use RWD to create a ‘virtual’ control arm from historical patient data. This is transformative in oncology and rare diseases, where recruiting for a placebo arm can be ethically or logistically impossible. The FDA has accepted submissions using SCAs, such as for the approval of blinatumomab for a rare form of leukemia. The process involves carefully selecting historical patients who match the trial’s eligibility criteria and using advanced statistical techniques, like propensity score matching, to minimize bias.
- Long-term monitoring and label expansion: RWD enables the tracking of treatment safety and effectiveness over years, uncovering long-term benefits and risks that short-term trials might miss. It can also provide the evidence needed to expand a drug’s approval to new patient populations or indications.
Informing Payers and Value-Based Reimbursement
Healthcare payers use real world data to determine which treatments deliver true value for money. RWD helps them understand how well a treatment works in the messy reality of clinical practice—considering factors like patient adherence and comorbidities—not just in the idealized setting of a controlled trial.
Health technology assessment (HTA) bodies now routinely incorporate RWD to ask tougher questions about comparative and cost-effectiveness. This allows for more accurate cost-effectiveness analysis based on actual usage patterns, adherence rates, and downstream healthcare costs (like reduced hospitalizations).
This evidence underpins the shift toward value-based care models, where providers and manufacturers are rewarded for positive patient outcomes. RWE makes innovative value-based agreements (VBAs) possible. In such a contract, a pharmaceutical company might provide rebates to a payer if their drug does not achieve pre-defined clinical endpoints in the payer’s patient population, with outcomes tracked using RWD.
Empowering Physicians and Improving Patient Outcomes
Real world data helps physicians answer the question: What’s the best treatment for this particular patient? Clinical decision support (CDS) tools powered by RWD can show how treatments have performed in thousands of patients with similar characteristics (age, comorbidities, genetic markers), moving beyond the population averages from trials.
Clinical practice guidelines become more dynamic and practical when informed by a continuous stream of RWE. Analyzing large-scale RWD also helps physicians and health systems understand disease progression and identify unmet needs by revealing treatment gaps or suboptimal outcomes in certain patient subgroups.
A clear example is the rapid generation of insights during health emergencies. Scientific research on COVID-19 outcomes using RWD among people with HIV informed treatment decisions when traditional research was too slow. This data-driven approach improves both individual care and population health management.
Navigating the Challenges and Ethical Considerations of RWD
Working with real world data presents significant challenges. The data is vast, messy, and requires careful attention to governance, interoperability, standardization, and ethics. Unlike the clean, prospectively collected data from clinical trials, RWD is a byproduct of routine care and can be incomplete, inconsistent, or contain errors.

Successfully navigating these issues requires a thoughtful, multi-faceted approach that balances innovation with responsibility. This means using robust methods to ensure findings are valid while upholding the highest ethical standards of privacy and fairness.
Ensuring Data Quality, Privacy, and Security
Data quality is not an absolute; it’s about fitness-for-purpose. Key challenges include data completeness (e.g., missing smoking status), accuracy (e.g., coding errors), and provenance (knowing the data’s origin and history). Data cleansing, validation, and quality assessment are critical first steps before any analysis.
Patient privacy is the most serious consideration. We are dealing with highly sensitive health information, and protecting it is a fundamental ethical and legal obligation. Regulations like HIPAA in the U.S. and GDPR in Europe set strict standards. Before use in research, RWD must be de-identified by removing personal identifiers (Safe Harbor Method) or be certified by an expert as having minimal re-identification risk (Expert Determination). Strong data security best practices, including encryption and access controls, are non-negotiable.
The Critical Role of Standardization and Interoperability
A major hurdle in leveraging RWD is that it is often siloed and recorded in different formats. This lack of interoperability makes it incredibly difficult to aggregate and analyze data from multiple sources. To address this, the industry is moving toward common data models (CDMs), which provide a standardized structure for health data.
One prominent example is the OMOP Common Data Model, which standardizes diverse datasets, allowing the same analysis script to be run across multiple databases. Another is FHIR, an API-based framework for real-time data exchange. The synergy between these standards is crucial for creating a connected, research-ready data ecosystem.
Overcoming Bias, Reproducibility, and Algorithmic Fairness
Because RWD is observational, it is susceptible to various biases that can distort findings. Key biases include confounding by indication (e.g., sicker patients get newer drugs) and selection bias (e.g., a study population isn’t representative of all patients). The reproducibility crisis in science is also amplified with RWD, as different analytical choices can lead to different conclusions.
Addressing these issues requires transparent methodologies and rigorous statistical methods. Techniques like target trial emulation and causal inference are used to mimic the structure of randomized trials, helping to control for confounders and isolate true treatment effects.
Algorithmic fairness is another critical consideration. As we use AI to analyze RWD, we must ensure our models do not perpetuate existing health disparities. This requires scrutinizing data for historical biases, building interpretable models, and monitoring outcomes across different demographic groups to ensure that RWD analytics benefit all patients equitably. At Lifebit, our federated platform is built on these principles, enabling powerful analytics without moving or exposing raw patient data, ensuring privacy, security, and fairness are never compromised.
The Future of RWD: Technology and Emerging Trends
The journey of real world data is just beginning, with predictive analytics, federated learning, and sophisticated data ecosystems ready to transform how we use health information. These emerging technologies are solving long-standing problems, like how to analyze messy data or collaborate on sensitive information without compromising privacy.

How AI and NLP are Enhancing the Use of Real World Data
Artificial Intelligence (AI) and Machine Learning (ML) are essential for handling the massive volume and variety of real world data. AI algorithms can spot patterns that would take human researchers years to identify, such as predicting patient responses to treatments or identifying early signs of adverse events.
Natural Language Processing (NLP) is open uping a treasure trove of information from unstructured text like physician notes, discharge summaries, and radiology reports. NLP reads these notes and automatically extracts critical details like diagnoses, symptoms, and treatment responses, turning qualitative observations into quantifiable data points.
Combining AI and NLP with RWD allows us to identify patient cohorts, track disease progression, and train AI models for drug development. For more, see our insights on How to Use Disease Real-World Population Data to Train AI Models.
Emerging Trends: Federated Analytics and Next-Generation Insights
The future of real world data is about smarter collaboration, and the biggest breakthrough is federated data networks. This model solves a key puzzle: how to analyze sensitive data without sharing it.
Instead of moving data to a central location, federated networks bring the analysis to the data. Patient information stays securely within hospital firewalls, while algorithms perform work locally and share only aggregated, non-identifiable results. This enables collaborative research on a global scale without exposing individual patient records.
Real-time analysis is another emerging frontier, moving us toward a world where doctors receive immediate, data-driven treatment recommendations. The integration of multi-omics data (genomics, proteomics, etc.) with clinical RWD is also creating richer datasets, bringing us closer to truly personalized medicine.
At Lifebit, we are actively building this future. Our platform enables federated data analysis, allowing researchers to securely access diverse RWD without moving sensitive information. Through our Trusted Research Environment, we are making secure, collaborative, real-time health research a reality. The future of real world data is federated, intelligent, and more promising than ever.
Frequently Asked Questions about Real World Data
Here are answers to the most common questions about real world data.
What is the main difference between RWD and RWE?
Think of it this way: RWD is the raw ingredients, and RWE is the finished meal.
Real world data (RWD) is the raw information collected from sources like electronic health records, insurance claims, and wearable devices. It’s the unprocessed data about what’s happening in everyday medicine.
Real World Evidence (RWE) is the clinical evidence and actionable insights generated from analyzing RWD. RWE is what informs decisions about health products and policies. In short, RWD tells us what happened, while RWE tells us what it means.
Is Real World Data reliable enough for regulatory approval?
Yes, increasingly so. Regulatory bodies like the FDA are developing robust frameworks to ensure RWD meets the quality standards needed for regulatory decisions. The FDA’s Real-World Evidence Program, created by the 21st Century Cures Act, outlines how RWE can support new drug approvals and post-approval studies.
The key is ensuring both sufficient data quality and rigorous analytical methods to account for potential biases. While RWD won’t completely replace randomized controlled trials (RCTs), it plays a vital role, especially in rare disease research, new indications for existing drugs, and long-term safety monitoring.
How is patient privacy protected when using RWD?
Patient privacy is fundamental and protected by multiple layers of security.
Regulatory compliance is the foundation. Laws like HIPAA in the U.S. and GDPR in Europe require that real world data be de-identified by removing personal identifiers before it can be used for research.
Advanced technologies like federated learning provide another layer of protection. This approach allows data to be analyzed without ever moving it from its secure source. The sensitive information stays behind institutional firewalls, and only aggregated, non-identifiable insights are shared.
At Lifebit, our federated platform is built on this principle, enabling secure collaboration while ensuring maximum privacy protection.
Conclusion
We’ve journeyed through real world data, from its basic definition to the AI and federated analytics shaping its future. RWD is no longer just a supplement to clinical trials—it’s revolutionizing every aspect of healthcare decision-making, from accelerating drug development to empowering physicians.
The real magic happens when we combine this data with the right technology and ethical governance. The future of evidence generation hinges on our ability to responsibly manage, analyze, and derive insights from this information while upholding stringent privacy protections.
At Lifebit, we are building the infrastructure to make this future possible. Our secure, federated AI platform empowers biopharma companies, governments, and public health agencies to harness the transformative power of real world data.
Through our Trusted Research Environment, Trusted Data Lakehouse, and R.E.A.L. platform, we securely connect global biomedical data to accelerate findies that improve patient care and improve human health worldwide.
The shift towards data-driven healthcare is already here. Organizations that accept real world data today will lead tomorrow’s medical breakthroughs.
Ready to harness the transformative potential of real world data for your organization? Learn how to leverage federated data for your research and join us in shaping the future of evidence-based healthcare. Together, we can turn data into findies and findies into better lives for patients everywhere.

