Precision Medicine, AI, and the Future of Personalized Health Care.
Why “One-Size-Fits-All” Medicine Is Costing Lives and Money
For decades, the practice of medicine has been built on a foundation of averages. A diagnosis would lead to a standard treatment protocol, a one-size-fits-all approach applied to a diverse population. While this model has saved countless lives, its limitations are becoming increasingly clear. It treats patients with similar symptoms the same way, ignoring the vast biological diversity that makes each person unique. This paradigm is not just inefficient; it’s actively costing lives and money. Precision medicine data analysis is the force dismantling this outdated model, ushering in an era where treatment is tailored to the individual.
This new approach works by integrating a wide array of diverse datasets—spanning electronic health records (EHRs), genomic sequences, proteomic data, and immunological profiles—to deliver personalized treatment strategies. Instead of relying on broad, population-based evidence, it uses the power of artificial intelligence (AI) and machine learning to uncover subtle patterns within an individual’s data that can predict disease risk, forecast progression, and determine the most effective treatment path. See the foundation in precision medicine.
The numbers tell a stark story. Up to 10% of global healthcare spending, amounting to hundreds of billions of dollars annually, is wasted on ineffective treatments. This waste stems from trial-and-error prescribing, where drugs are given to patients who will not respond. For example, only about 50% of patients with depression respond to the first antidepressant they are prescribed. Similarly, common statins are effective for only a fraction of the individuals who take them. These inefficiencies lead to prolonged illness, significant costs from adverse drug reactions, and a heavy burden on both patients and healthcare systems. The reality is that while behavioral and environmental factors account for 60% of health outcomes and genetics for 30%, most treatment decisions are made in a data vacuum, ignoring this crucial context.
AI is the key to unlocking this data. Over a lifetime, each person generates a staggering volume of health-related information—equivalent to 300 million books. This isn’t just clinical data from hospital visits; it includes genomic blueprints, real-time physiological data from wearable devices (heart rate, sleep patterns, activity levels), and environmental exposure information. Without AI-powered analysis, this treasure trove of personalized health information sits fragmented and unused. As Maria Chatzou Dunford, CEO of Lifebit, I’ve spent over 15 years developing federated platforms that turn this raw, multi-modal data into actionable clinical insights. These platforms make it possible to conduct secure, compliant precision medicine research across global healthcare networks, finally enabling us to see the whole patient and treat them with the precision they deserve.
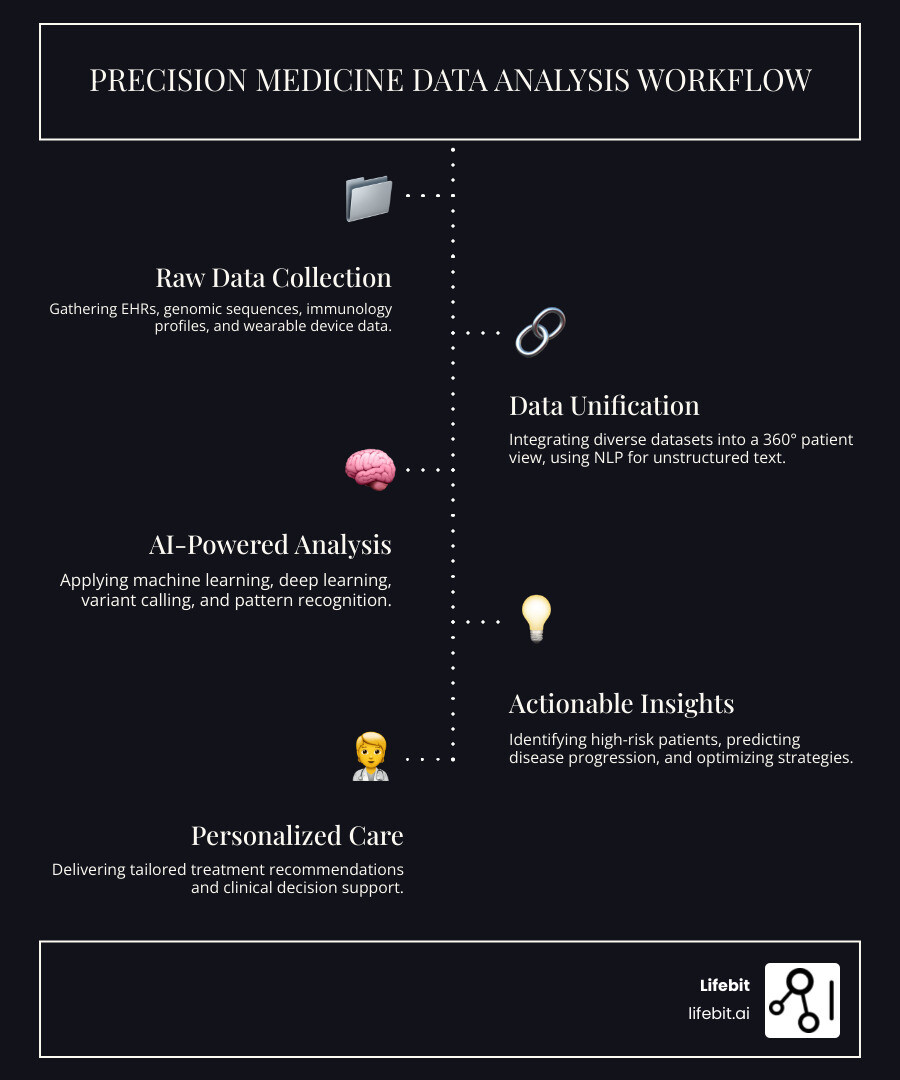
Unify Your Data: Build a 360° Patient View and End Data Silos
Critical health information, the very data needed for precision medicine, is trapped in disconnected silos. Your electronic health record (EHR) holds clinical notes, but your full genomic sequence is stored in a separate research database. Your immunology lab results exist in another system entirely, and real-time data from your smartwatch is on a proprietary cloud. This fragmentation is the single greatest barrier to personalized care. It forces clinicians to make decisions with an incomplete puzzle, leading to the generic, trial-and-error care we aim to leave behind. Imagine a rheumatologist treating an autoimmune patient; without access to the patient’s genetic markers for drug metabolism alongside their clinical history of flare-ups, the choice of a powerful biologic drug is a shot in the dark.
EHRs, genomics, and immunology data are the holy trinity of precision medicine, each providing a unique and critical layer of the patient’s story.
- Electronic Health Records (EHRs): This is the longitudinal narrative of a patient’s health journey. It contains structured data like lab values, medication lists, and diagnostic codes, but its richest insights are often locked in unstructured clinical notes. This is where Natural Language Processing (NLP) becomes essential. NLP algorithms can read and interpret free-text notes, extracting crucial information like symptom severity, disease progression, and social determinants of health, transforming messy text into clean, analyzable data. Learn more about NLP here.
- Genomic Data: Our DNA holds the blueprint for how our bodies function. Whole-genome or exome sequencing reveals genetic variants that can predispose us to diseases, influence how we metabolize drugs (pharmacogenomics), and determine whether a targeted therapy will be effective. For example, identifying the HLA-B*57:01 allele is critical to prevent a potentially fatal hypersensitivity reaction to the HIV drug abacavir.
- Immunological Profiles: Generated by techniques like flow cytometry and single-cell sequencing, this data provides a high-resolution snapshot of a patient’s immune system. It reveals the types, states, and functions of immune cells, explaining why some individuals develop autoimmune diseases or why a cancer patient might respond to immunotherapy while another does not.
A unified approach, such as a research environment or Data Commons, is needed to connect these disparate sources. However, the sensitive nature of patient data makes centralization a logistical and ethical nightmare, fraught with privacy risks and governance challenges. Federated analysis elegantly solves this problem by bringing the analysis to the data, a concept central to federated learning. Instead of moving petabytes of sensitive data to a central location, analytical algorithms are sent to each secure, siloed data source. The analysis is performed locally, behind the institution’s firewall, and only the aggregated, non-identifiable results are returned. This model, which adheres to FAIR (Findable, Accessible, Interoperable, Reusable) data principles, allows researchers to spot complex patterns across multiple global datasets without ever exposing or moving the underlying patient data. It enables the creation of a true 360° biological story for each patient, paving the way for truly personalized care.
Learn how Lifebit unifies health data for precision medicine.
AI in Action: How Smart Analysis Turns Data into Life-Saving Decisions
Artificial intelligence is the engine that drives precision medicine, transforming massive, complex datasets into actionable clinical decisions. Instead of reacting to symptoms after they appear, AI enables a proactive approach, helping us predict who will get sick, when they might need an intervention, and which specific treatments will work best for them—often years before traditional diagnostic methods would catch a problem. This represents a fundamental shift from reactive care to P4 healthcare: predictive, preventive, personalized, and participatory.
- Predictive: AI models analyze risk factors across genomic, clinical, and lifestyle data to forecast disease onset. For example, AI can analyze EHRs to spot high-risk patients for autoimmune diseases up to five years before a traditional diagnosis.
- Preventive: With accurate predictions, clinicians can intervene earlier with preventative strategies, such as lifestyle changes, targeted screenings, or prophylactic treatments, potentially stopping a disease before it ever fully develops.
- Personalized: AI ensures that the treatment fits the patient, not just the disease. It tailors drug choice, dosage, and therapeutic strategy to an individual’s unique biological makeup.
- Participatory: Patients become active partners in their own healthcare, empowered by access to their data and a clearer understanding of their personal health risks and treatment options.
This is made possible by different families of AI models. Machine learning (ML) models, such as random forests and support vector machines, excel at finding patterns in structured data like lab results, diagnostic codes, and medication history to identify high-risk patient cohorts. Deep learning (DL), a more advanced subset of ML using neural networks, goes further by analyzing raw, unstructured data. Convolutional Neural Networks (CNNs) can interpret medical images like X-rays and MRIs with superhuman accuracy, while other architectures can decode the complex language of genetic sequences.
See how AI is changing health data analysis.
Predict and Personalize: AI’s Impact on Autoimmune Disease
Autoimmune diseases like rheumatoid arthritis (RA), which are notoriously heterogeneous and difficult to treat, are a prime example of AI’s impact. Machine learning algorithms can now predict therapy response before a patient even starts a costly and potentially toxic treatment. By analyzing a combination of clinical data (e.g., inflammation markers like CRP) and genetic data, models can identify which patients will respond to first-line drugs like methotrexate versus those who would benefit from an immediate move to a biologic TNF inhibitor, saving months of ineffective treatment.
Furthermore, AI can:
- Model mortality risk: Algorithms like Random Survival Forests use longitudinal clinical data—including lab values, comorbidities, and patient-reported outcomes—to predict which RA patients face a higher mortality risk, enabling clinicians to allocate more intensive monitoring and care to those who need it most.
- Quantify joint damage: Deep learning-based CNNs analyze hand X-rays to automatically detect and score joint space narrowing and bone erosion. This provides an objective, reproducible measurement of disease progression, replacing subjective scoring methods and allowing for more precise tailoring of therapeutic strategies.
Decode the Genome and Map Immunity with AI
The challenge with genomic data isn’t collection; it’s interpretation. A single human genome contains billions of data points. AI has revolutionized this analytical process:
- Variant Calling: AI tools like Google’s DeepVariant reframe the task of identifying genetic mutations from sequencing reads as an image classification problem. By treating the data like pictures, it leverages powerful CNNs to identify genetic variants with superior accuracy compared to traditional statistical methods.
- Pathogenicity Prediction: Once a variant is found, the key question is whether it’s harmful. Models like DeepMind’s AlphaMissense, an evolution of the protein-folding model AlphaFold, predict the structural and functional impact of a variant. This helps clinicians distinguish harmless genetic quirks from disease-causing mutations, focusing attention on what truly matters.
- Splicing Analysis: Some variants don’t alter a protein directly but disrupt how a gene is processed (spliced). Tools like SpliceAI use deep neural networks to accurately predict how variants will affect splicing, catching disease-causing problems that other analyses would miss.
How Lifebit accelerates genomic insights.
Similarly, in immunology, where a single blood sample analyzed with mass cytometry can generate millions of data points per patient, AI is essential for making sense of the complexity.
- Visualizing Complexity: Dimensionality reduction techniques like UMAP and t-SNE transform high-dimensional immune data into intuitive 2D or 3D maps. These visualizations allow researchers to literally see the immune system, revealing hidden cell populations or shifts in cell states that are associated with disease.
- Automating Identification: AI automates the laborious and subjective process of identifying immune cell types (known as “gating” in flow cytometry). This provides a consistent, objective, and scalable analysis that can reliably detect rare but clinically significant cell populations.
By applying these AI-enabled techniques, we gain an unprecedented, multi-faceted view into individual biological responses, paving the way for truly personalized diagnostics and therapies.
From Lab to Life: Real-World Wins with AI-Driven Precision Medicine
The theoretical promise of AI in precision medicine is now a clinical reality. By integrating and analyzing health records, genetics, and immunology data through federated platforms, we are revolutionizing patient care, particularly in complex, heterogeneous diseases like rheumatoid arthritis and cancer. The impact is not incremental; it is a fundamental transformation of diagnostics, treatment, and research.
The difference between the old way and the new, AI-driven approach is staggering:
| Feature | Traditional Approach | AI-Driven Approach |
|---|---|---|
| Diagnostic Time | Months to years of specialist referrals and tests. | Weeks or days, with AI models predicting risk years in advance from EHR data. |
| Treatment Selection | Trial-and-error; 30-40% response rate to first therapy. | Data-driven; predictive models match patients to drugs, boosting response rates to 70-85%. |
| Outcome Prediction | Based on clinical intuition and population-level statistics. | Personalized risk scores predict disease progression and mortality with high accuracy. |
| Data Utilization | 97% of health data is unused; insights are locked in silos. | Federated analysis unlocks siloed data for a holistic patient view without compromising privacy. |
| Patient Experience | A long, frustrating journey of uncertainty and ineffective treatments. | An empowered, proactive journey with clear, personalized care paths. |
These metrics represent a paradigm shift with tangible benefits:
- Improved Diagnostic Accuracy and Speed: Consider a patient with vague, systemic symptoms. Traditionally, they face a “diagnostic odyssey” that can last for years. In the new paradigm, an AI model analyzing their EHR flags a subtle pattern of lab results and symptoms, suggesting a high risk for a specific autoimmune disease. This triggers an immediate, targeted referral, shrinking the diagnostic timeline from years to weeks and allowing treatment to begin before irreversible damage occurs.
- Personalized Treatment Strategies: Instead of cycling through standard treatments, AI enables true personalization. In oncology, a patient with non-small cell lung cancer can have their tumor’s genomic profile analyzed. An AI platform can identify a rare ALK fusion, for which a targeted inhibitor like Alectinib offers a >80% response rate, compared to <30% for traditional chemotherapy in that patient. This is the core of precision medicine: the right drug, for the right patient, at the right time.
- Accelerated Research and Development: AI is drastically reshaping how new therapies are developed. By analyzing real-world data, AI can help design smarter clinical trials. It identifies ideal patient cohorts, predicts potential placebo-responders, and can even create “synthetic” control arms from existing data. This allows trials to be smaller, faster, and more likely to succeed, bringing life-saving drugs to the market years earlier and at a fraction of the cost.
Overcoming the Hurdles to Implementation
Despite the immense potential, the path to widespread adoption is not without challenges. Key hurdles include:
- Data Quality and Standardization: AI models are only as good as the data they are trained on. Inconsistent data formats, missing information, and biases in historical data must be addressed.
- Regulatory and Ethical Frameworks: AI-based diagnostic tools are often classified as medical devices, requiring rigorous validation and regulatory approval from bodies like the FDA. Clear ethical guidelines are needed for data privacy, consent, and algorithmic fairness.
- Clinical Workflow Integration: For AI tools to be effective, they must be seamlessly integrated into a clinician’s daily workflow, providing clear, interpretable, and actionable insights at the point of care without causing alert fatigue.
This move from reactive to predictive healthcare is happening now, powered by precision medicine data analysis. By tackling these challenges head-on, we can ensure that patients spend less time suffering, healthcare systems reduce waste, and doctors are empowered to deliver the truly personalized care that was once the domain of science fiction.
AI in Action: How Smart Analysis Turns Data into Life-Saving Decisions
Instead of treating symptoms after they appear, AI helps us predict who will get sick, when they’ll need intervention, and which treatments will work best—often years before traditional methods.
Machine learning (ML) models excel at finding patterns in structured data like lab results and medication history to identify high-risk patients. Deep learning (DL) goes further, analyzing raw, unstructured data like medical images and genetic sequences. Together, they enable a shift from reactive medicine to P4 healthcare: predictive, preventive, personalized, and participatory. For example, AI can analyze EHRs to spot high-risk patients for autoimmune diseases up to five years before a traditional diagnosis.
See how AI is changing health data analysis.
Predict and Personalize: AI’s Impact on Autoimmune Disease
Autoimmune diseases like rheumatoid arthritis (RA) are notoriously difficult to treat. AI is changing this. Machine learning algorithms can now predict therapy response before a patient even starts treatment. By analyzing clinical and genetic data, models identify which patients will respond to specific drugs like methotrexate, helping doctors skip ineffective treatments.
Furthermore, AI can:
- Model mortality risk: Random Survival Forest algorithms use clinical data to predict which RA patients face higher mortality risk, allowing for more intensive care.
- Quantify joint damage: AI analyzes X-rays to objectively measure joint damage, helping tailor therapeutic strategies and monitor treatment effectiveness with a precision that subjective assessments lack.
Decode the Genome and Map Immunity with AI
The challenge with genomic data isn’t collection; it’s analysis. AI has revolutionized this process:
- Variant Calling: AI tools like DeepVariant treat DNA sequencing data like images to identify genetic variants with superior accuracy.
- Pathogenicity Prediction: Models like AlphaMissense analyze protein structures to determine if a genetic variant is harmless or disease-causing, helping doctors focus on what matters.
- Splicing Analysis: Tools like SpliceAI predict how variants affect gene processing, catching problems that traditional analysis might miss.
How Lifebit accelerates genomic insights.
Similarly, in immunology, where a single sample can generate millions of data points, AI is essential.
- Visualizing Complexity: Techniques like UMAP and t-SNE transform high-dimensional immune data into understandable 2D or 3D maps, revealing hidden cell populations.
- Automating Identification: AI automates the identification of immune cell types, providing consistent and objective analysis that can detect rare, disease-associated cells.
By applying these AI-enabled techniques, we gain unprecedented insight into individual biological responses, paving the way for truly personalized diagnostics and therapies.
From Lab to Life: Real-World Wins with AI-Driven Precision Medicine
The theoretical promise of AI in precision medicine is now a clinical reality. By integrating health records, genetics, and immunology data, we are revolutionizing patient care, especially in complex diseases like rheumatoid arthritis.
The difference between the old way and the new way is staggering:
| Feature | Traditional Approach | AI-Driven Approach |
|---|---|---|
| Diagnostic Time | 6-12 months for accurate RA diagnosis | AI can identify high-risk patients up to 5 years earlier through EHR analysis |
| Treatment Accuracy | Trial-and-error approach with 30-40% response rates | Precision medicine data analysis predicts therapy response with 70-80% accuracy |
| Outcome Prediction | Limited ability to forecast disease progression | Machine learning models accurately predict mortality risk and joint damage progression |
These aren’t just incremental improvements—they represent a fundamental shift.
- Improved Diagnostic Accuracy: AI models catch disease patterns earlier, meaning patients get the right treatment before irreversible damage occurs.
- Personalized Treatment Strategies: Instead of trial-and-error, AI predicts which therapies will work for an individual’s biological profile, forecasting response with up to 80% accuracy.
- Accelerated Research: AI helps design better clinical trials by identifying the right patients, meaning life-saving drugs reach the market faster.
This move from reactive to predictive healthcare is happening now, powered by precision medicine data analysis. Patients spend less time suffering, healthcare systems reduce costs, and doctors can deliver truly personalized care.
AI in Action: How Smart Analysis Turns Data into Life-Saving Decisions
Instead of treating symptoms after they appear, AI helps us predict who will get sick, when they’ll need intervention, and which treatments will work best—often years before traditional methods.
Machine learning (ML) models excel at finding patterns in structured data like lab results and medication history to identify high-risk patients. Deep learning (DL) goes further, analyzing raw, unstructured data like medical images and genetic sequences. Together, they enable a shift from reactive medicine to P4 healthcare: predictive, preventive, personalized, and participatory. For example, AI can analyze EHRs to spot high-risk patients for autoimmune diseases up to five years before a traditional diagnosis.
See how AI is changing health data analysis.
Predict and Personalize: AI’s Impact on Autoimmune Disease
Autoimmune diseases like rheumatoid arthritis (RA) are notoriously difficult to treat. AI is changing this. Machine learning algorithms can now predict therapy response before a patient even starts treatment. By analyzing clinical and genetic data, models identify which patients will respond to specific drugs like methotrexate, helping doctors skip ineffective treatments.
Furthermore, AI can:
- Model mortality risk: Random Survival Forest algorithms use clinical data to predict which RA patients face higher mortality risk, allowing for more intensive care.
- Quantify joint damage: AI analyzes X-rays to objectively measure joint damage, helping tailor therapeutic strategies and monitor treatment effectiveness with a precision that subjective assessments lack.
Decode the Genome and Map Immunity with AI
The challenge with genomic data isn’t collection; it’s analysis. AI has revolutionized this process:
- Variant Calling: AI tools like DeepVariant treat DNA sequencing data like images to identify genetic variants with superior accuracy.
- Pathogenicity Prediction: Models like AlphaMissense analyze protein structures to determine if a genetic variant is harmless or disease-causing, helping doctors focus on what matters.
- Splicing Analysis: Tools like SpliceAI predict how variants affect gene processing, catching problems that traditional analysis might miss.
How Lifebit accelerates genomic insights.
Similarly, in immunology, where a single sample can generate millions of data points, AI is essential.
- Visualizing Complexity: Techniques like UMAP and t-SNE transform high-dimensional immune data into understandable 2D or 3D maps, revealing hidden cell populations.
- Automating Identification: AI automates the identification of immune cell types, providing consistent and objective analysis that can detect rare, disease-associated cells.
By applying these AI-enabled techniques, we gain unprecedented insight into individual biological responses, paving the way for truly personalized diagnostics and therapies.
From Lab to Life: Real-World Wins with AI-Driven Precision Medicine
The theoretical promise of AI in precision medicine is now a clinical reality. By integrating health records, genetics, and immunology data, we are revolutionizing patient care, especially in complex diseases like rheumatoid arthritis.
The difference between the old way and the new way is staggering:
| Feature | Traditional Approach | AI-Driven Approach |
|---|---|---|
| Diagnostic Time | Weeks to months of testing and specialist referrals | Days with predictive algorithms analyzing EHR patterns |
| Treatment Accuracy | 30-40% initial therapy success rate | 70-85% success with genomic and clinical data integration |
| Outcome Prediction | Limited to clinical experience and basic lab results | Machine learning models predict 5-year disease progression |
These aren’t just incremental improvements—they represent a fundamental shift.
- Improved Diagnostic Accuracy: AI models catch disease patterns earlier, meaning patients get the right treatment before irreversible damage occurs.
- Personalized Treatment Strategies: Instead of trial-and-error, AI predicts which therapies will work for an individual’s biological profile, forecasting response with up to 80% accuracy.
- Accelerated Research: AI helps design better clinical trials by identifying the right patients, meaning life-saving drugs reach the market faster.
This move from reactive to predictive healthcare is happening now, powered by precision medicine data analysis. Patients spend less time suffering, healthcare systems reduce costs, and doctors can deliver truly personalized care.

