More Than Medicine: Why Patient Support Matters in Clinical Research

Stop 40% Trial Dropouts: The Patient Support Playbook That Saves Years and Millions
Clinical trial patient support refers to the services and resources that help participants steer their trial journey. This includes everything from concierge services and logistical assistance (travel, scheduling) to financial support, digital tools, and educational resources.
The stakes are incredibly high. A staggering 40% of patients drop out of clinical trials before completion, a reality that doesn’t just delay breakthroughsit can derail them entirely.
As Preeti Gupta, a physician diagnosed with ovarian cancer, shared about her trial search: “Finding the right clinical trial is so hard that I have cried trying to figure out what to do.” Her story underscores why comprehensive support is essential for making research accessible and sustainable.
The cost of poor patient support is staggering. Bringing a new therapy to market can take over a decade and cost more than $2 billion. When patients drop out, these timelines and costs multiply.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. For over 15 years, we’ve focused on improving clinical trial patient support by changing how organizations access and analyze patient data on secure, federated platforms. Our experience has proven that robust patient support is the critical factor separating trial success from failure.
Clinical trial patient support terms you need:
- clinical trial recruitment strategies
- digital clinical trial recruitment
- clinical trial recruitment digital case study
Deploy Concierge Support to Hit 85%+ Retention and Slash Dropouts
Imagine you’re dealing with a serious health condition. The diagnosis alone is overwhelming, and now a clinical trial offers hope, but you’re immediately buried in logistical complexities: scheduling, travel, paperwork, and costs. This is where clinical trial patient supportspecifically concierge services and contact centersbecomes a game-changer. They act as a single, reliable point of contact and a personal advocate, navigating the complexities of clinical research alongside you so you can focus on your health.
These services provide a crucial human connection, offering everything from appointment scheduling and travel booking to emotional support and financial guidance. The impact is remarkable: while trials without proper support see a devastating 40% dropout rate, comprehensive concierge services can boost retention to over 85%. This human-centric approach transforms the trial experience from a daunting obligation into a supported journey.
Enrollment and Onboarding Assistance
Starting a trial can be confusing, and the informed consent process is often the first major hurdle. A good concierge service turns that confusion into clarity. They don’t just hand you a 50-page document; they walk you through every detail. They can help explain complex medical terminology, clarify concepts like randomization and blinding, and ensure you fully understand the trial’s purpose, procedures, potential risks, and benefits. By patiently answering every question and setting realistic expectations about time commitments and potential side effects from day one, they build a foundation of trust and psychological safety that is essential for long-term participation.
Logistical Support for Participants
Concierge services turn logistical nightmares into seamless experiences. They handle the full spectrum of travel and accommodation needs, from booking flights and hotels to arranging ground transportation. For participants with greater needs, this can extend to coordinating with home health nurses, arranging for childcare during appointments, or even assisting with visa applications for international trials. Instead of spending hours on the phone managing logistics, you receive a simple, clear itinerary. This comprehensive support removes the significant stress and hidden labor of trial participation, allowing you to conserve your energy and focus entirely on your health and treatment.
Financial Assistance and Reimbursements
Financial worries should never force someone out of a potentially life-saving trial. Concierge services tackle these concerns head-on by providing clear, upfront information about what costs the trial sponsor covers. They act as your financial navigator, managing the entire reimbursement process so you aren’t left chasing paperwork. The best programs have moved beyond the slow reimbursement model and now use direct-pay systems with reloadable expense cards. This means you aren’t paying out-of-pocket for trial-related expenses like travel, meals, or lodging. This approach not only reduces stress but also promotes equity, ensuring that a patient’s financial situation is not a barrier to accessing cutting-edge research.
The Human Element: Building Trust and Empathy
While technology plays a growing role, the core of a successful concierge service is its human element. Concierge staff are more than just coordinators; they are trained professionals skilled in active listening, cultural competency, and empathetic communication. They understand the emotional toll of a serious illness and the anxieties of participating in research. They become a trusted confidanta consistent, supportive voice a patient can turn to with concerns big and small. This relationship is what truly drives retention. When participants feel seen, heard, and valued as individuals rather than just data points, they are far more likely to remain engaged and committed throughout the trial’s duration.
Post-Trial Follow-Up and Communication
The best support programs don’t end with the last visit. Quality concierge services maintain contact long after the trial concludes, playing a vital role in sharing study results and guiding you toward continued care options. They also gather your feedback through post-trial surveys to identify pain points and improve the experience for future participants. This transforms you from a temporary study subject into a valued, lifelong member of a research community, fostering goodwill and encouraging future participation in clinical research.
Turn 40% Dropouts into 85%+ Retention with Patient Apps, ePROs, and Telehealth
Imagine a trial participant receiving a gentle, personalized reminder on her phone to log her symptoms. She opens a user-friendly app and completes her electronic diary in two minutes, a task that used to involve cumbersome paper forms. This is the power of digital tools in modern clinical trial patient support. With a staggering 40% of patients dropping out of trials, technology is no longer a luxury; it is an essential component for keeping participants engaged, collecting high-quality data, and keeping trials on track.
The results are clear. Patient engagement platforms rooted in behavioral science have been shown to boost retention rates to over 85%, with some achieving patient satisfaction rates as high as 96%. These digital tools are the backbone of decentralized clinical trials (DCTs) and hybrid models, which are rapidly becoming the new standard. By bringing the trial to the patient, these technologies give participants the flexibility they need, reducing the burden of frequent site visits while simultaneously improving the consistency and quality of the data collected.
How Technology Improves Clinical Trial Patient Support
Technology makes trial participation more personal, convenient, and accessible. It empowers patients and provides researchers with richer insights.
- Mobile apps and patient portals act as a digital front door to the trial. They feature integrated calendars for appointments, secure messaging with the study team, push notifications for medication reminders, and a library of educational content about the trial and the patient’s condition.
- Wearable devices and sensors (e.g., smartwatches, continuous glucose monitors, smart scales) passively collect a continuous stream of objective, real-world data like heart rate, sleep patterns, and activity levels. This reduces the burden of manual logging and provides a far more accurate picture of a patient’s health than sporadic in-clinic measurements.
- Telehealth visits offer a convenient alternative to in-person check-ins for routine follow-ups, adverse event reporting, and specialist consultations. This saves patients significant time and money related to travel and time off work.
- Gamification uses game-like elements, such as progress bars, badges, and rewards, to make routine tasks like medication adherence and diary completion feel less like a chore and more engaging.
- Electronic Patient-Reported Outcomes (ePROs) replace paper forms with user-friendly digital versions. They use features like time-stamping and conditional branching logic (e.g., if a patient reports a headache, the app automatically asks follow-up questions about severity and duration), leading to cleaner, more complete, and more accurate data.
- AI-powered chatbots provide instant, 24/7 answers to common, non-medical questions (e.g., “When is my next appointment?” or “How do I submit an expense?”), freeing up human staff to focus on more complex patient needs.
- Blockchain technology is an emerging tool that can help patients securely manage their own medical records and control who has access to their data, enhancing transparency and trust.
These tools don’t replace human connectionthey augment and improve it, creating a more responsive and supportive trial experience.
Best Practices for Digital Engagement
Effective digital engagement isn’t about deploying the flashiest technology; it’s about thoughtfully designing a system that serves patients better.
Key practices include providing personalized content relevant to the patient’s journey, using real-time data insights to proactively identify and address potential issues (like missed medications), and ensuring a simple, intuitive, and user-friendly design. Rock-solid security and privacy are non-negotiable to build and maintain the deep trust required for patients to share their sensitive health data.
Bridging the Digital Divide: Ensuring Equity and Inclusion
A critical best practice is to ensure that technology promotes inclusivity rather than creating barriers. Not all patients have access to a smartphone or a reliable internet connection, and digital literacy varies widely. To ensure digital equity, sponsors should adopt a “provisioning” model, providing participants with pre-configured devices (e.g., a smartphone or tablet) and a data plan for the duration of the trial. This must be paired with robust, 24/7 technical support to help users with setup and troubleshooting. Furthermore, platforms must be designed for accessibility, with features like large fonts, simple navigation, and multi-language support to accommodate all users, regardless of age, ability, or tech-savviness. Finally, as the National Institute for Health Research emphasizes, it’s vital to “close the loop” by sharing study results and showing participants how their digital contributions made a difference. This transparency builds trust and encourages future engagement.
Activate Caregivers to Boost Adherence and SafetyYour Hidden Retention Edge
Behind almost every trial participant is an invisible support network of family, friends, and caregivers. While professional clinical trial patient support provides essential logistical and financial help, this personal network provides the day-in, day-out emotional and practical backbone that is crucial for navigating the ups and downs of a trial. Recognizing and formally integrating these caregivers into the support plan is a powerful strategy for improving patient adherence, safety, and retention.
This support ecosystem also extends beyond the home. Broad public engagement builds trust in the research system as a whole, encouraging more people to see research as a collaborative partnership. Organizations like Clinical Trials Ontario have demonstrated that involving patients, caregivers, and the public in trial design creates a more ethical and effective research environment, changing the paradigm from research that happens to patients into research that happens with them.

Key Considerations for Caregivers
Caregivers are active partners in the research process, and empowering them with information is key. With the patient’s permission, they should be included in key discussions.
- Understanding the informed consent document: Caregivers should be invited to the consent discussion to understand the trial’s schedule, procedures, and potential risks. This allows them to know what to expect and what to monitor.
- Monitoring and reporting side effects: Caregivers are uniquely positioned to notice subtle changes in a patient’s health, mood, or behavior that the patient might miss or dismiss. Their observations provide an invaluable early warning system for adverse events.
- Maintaining open communication: A strong, three-way line of communication between the patient, caregiver, and study team creates a robust safety net, ensuring that concerns are addressed quickly.
- Practicing self-care: Caregiving is demanding. It is essential for caregivers to manage their own emotional and physical well-being to avoid burnout. The Caregiver Action Network offers valuable resources, including a guide on Supporting Your Loved One in a Clinical Trial.
The Caregiver’s Expanded Role in Clinical Trial Patient Support
Caregivers often become unsung heroes, acting as an extension of the research team. Their multifaceted role includes:
- Observing and reporting: Providing crucial observations about the patient’s daily condition, which offers a more complete picture than can be gathered in brief clinic visits.
- Managing complex schedules: Juggling medication timings, dietary restrictions, and frequent appointments specific to the trial protocol.
- Handling logistics: Arranging transportation and accompanying the patient to lengthy appointments.
- Acting as an advocate: Asking clarifying questions the patient may forget, ensuring the patient’s concerns are heard, and reinforcing information from the study team.
- Taking detailed notes: Capturing complex information during appointments to help the patient remember instructions and next steps.
- Providing emotional stability: Helping the patient manage the anxiety, frustration, and uncertainty that can accompany a clinical trial.
Integrating Caregivers into the Trial Protocol to Prevent Burnout
Forward-thinking trial sponsors are moving beyond passive acknowledgment and are now actively integrating caregivers into the support structure. This formal recognition is crucial for preventing caregiver burnout, which is a significant indirect risk to patient retention. Strategies include:
- Dedicated Caregiver Resources: Providing caregivers with their own educational materials, FAQs, and contact lists so they feel equipped for their role.
- Direct Lines of Communication: Offering caregivers access to a dedicated support line or coordinator to ask their own questions without having to go through the patient.
- Financial and Logistical Support: Reimbursing caregivers for their own expenses, such as parking, meals during long clinic days, or even lost wages, acknowledging the real costs associated with their role.
- Peer Support Networks: Connecting caregivers with others in similar situations to share experiences and advice, reducing feelings of isolation.
A caregiver’s dedication supports not just one person; their active, recognized partnership strengthens the entire research process and contributes to breakthroughs that could help countless others.
6 Myths Costing You Patients and TimeDebunk Them and Hit 85%+ Retention
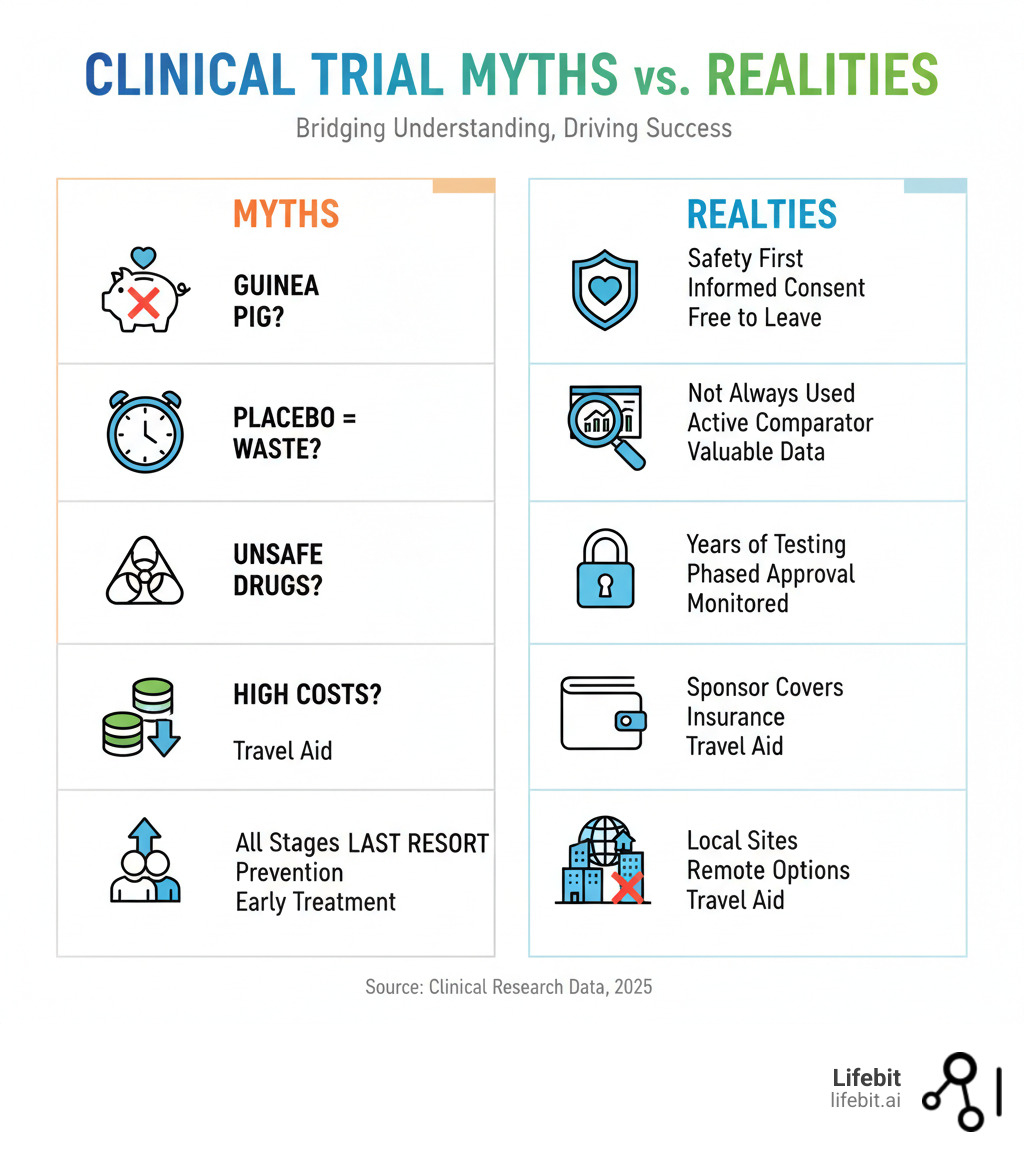
“I don’t want to be a guinea pig.” This common fear, rooted in pervasive myths and misconceptions about clinical research, prevents many people from participating in potentially life-saving trials. A lack of clear, accessible information creates a vacuum that is easily filled by fear and mistrust.
Effective clinical trial patient support is about proactively replacing fear with facts. When patients and their families feel informed, respected, and supported, they are far more likely to enroll and complete trials. This directly tackles the industry’s chronic 40% dropout rate, with well-supported trials now seeing retention rates soar above 85%. Let’s debunk the most common myths that stand between patients and breakthrough treatments.
Debunking Common Myths About Trial Participation
- The “Guinea Pig” Myth: This is perhaps the most damaging myth. In reality, investigational drugs undergo years of rigorous laboratory and animal testing before they are ever considered for human trials. Every trial in the U.S. must be approved and monitored by an Institutional Review Board (IRB), an independent committee of physicians, statisticians, and community members whose primary job is to protect participant rights and welfare. Participants receive full details in the informed consent process and can leave a trial at any time, for any reason.
- The Placebo Myth: The idea of receiving a “sugar pill” instead of real treatment is a major concern. However, placebos are not used when it would be unethical to deny a patient effective therapy. In trials for serious diseases like cancer or heart disease, participants typically receive either the new treatment or the current best available treatment (known as the “standard of care”). No one is ever left untreated.
- The Safety Myth: While no medical treatment is 100% risk-free, clinical trials are designed with safety as the highest priority. Trials are conducted in multiple phases (Phase I, II, and III) to build up safety and efficacy data gradually and cautiously. Furthermore, most trials are monitored by an independent Data and Safety Monitoring Board (DSMB), which periodically reviews the data to identify any emerging safety concerns and has the authority to recommend stopping a trial if necessary.
- The Cost Myth: Participating in a clinical trial should not create a financial burden. The trial sponsor covers all research-related costs, including the investigational drug, study-specific tests, and any extra doctor visits. Participants are also often reimbursed for expenses like travel, parking, and lodging, and may receive a stipend for their time and effort. The informed consent document provides a clear breakdown of all financial aspects.
- The “Last Resort” Myth: Many people believe trials are only for patients who have exhausted all other treatment options. In fact, trials recruit patients at all stages of a disease. There are trials for newly diagnosed patients, trials for prevention in high-risk individuals (e.g., vaccine trials), and trials focused on improving quality of life or managing symptoms.
- The Geography Myth: While many trials have historically been concentrated at major academic medical centers, this is rapidly changing. The rise of decentralized clinical trials (DCTs) and hybrid models means many trial activities can now be done remotely. A local lab can draw blood, a home health nurse can conduct check-ups, and the study drug can be shipped directly to a patient’s home, making participation possible regardless of geographic location.
The Impact on Trial Outcomes and Commercialization
When support services and clear communication bust these myths, the business impact is profound.
- Improved retention and faster timelines: High retention means trials complete on schedule. Delays are incredibly expensive, with some estimates putting the cost at between $600,000 and $8 million per day in lost revenue for a potential blockbuster drug. Avoiding dropouts is one of the most effective ways to protect the trial’s timeline and budget.
- Higher quality data: Engaged, informed participants are more likely to adhere to the study protocol (e.g., taking medication correctly, completing diaries). When participants drop out, it creates missing data, which weakens the statistical power of the study and can raise difficult questions from regulators like the FDA and EMA during the approval process. High retention leads to a cleaner, more robust data package.
- Improved drug approval prospects and greater public trust: A well-run trial with high retention and strong data is more likely to result in a successful regulatory submission. Beyond a single drug, positive trial experiences build public trust in the research enterprise, creating a sustainable cycle of participation, innovation, and medical advancement.
Investing in clinical trial patient support isn’t just an ethical obligationit’s one of the smartest business decisions a sponsor can make.
Clinical Trial Support FAQ: Cut Confusion, Costs, and Dropouts
Considering a clinical trial? It’s natural to have questions. Here are answers to the most common concerns about clinical trial patient support, based on our experience at Lifebit.
What types of support are typically available to trial participants?
Modern trials offer a wide range of support to make participation as smooth as possible. This typically includes:
- Logistical Support: Appointment reminders, travel arrangements, and accommodation coordination.
- Financial Assistance: Reimbursement for expenses like travel and parking, plus stipends for your time. Many programs use reloadable expense cards to simplify the process.
- Educational Resources: Clear, jargon-free information about the trial and treatment.
- Digital Tools: Mobile apps for tracking symptoms, receiving reminders, and communicating with your study team.
- A Dedicated Study Coordinator: Your primary contact and personal guide for the trial.
- Emotional and Caregiver Support: Resources for you and your family.
Who is the main point of contact for questions during a clinical trial?
Your study coordinator is your primary point of contact for most day-to-day questions. They are your guide and advocate throughout the trial.
For complex medical questions, the coordinator will connect you with the Principal Investigator (PI), the lead doctor overseeing the study. Many trials also offer contact center support for after-hours questions. You will always have a clear line of communication and an emergency contact card for immediate medical needs.
Are there out-of-pocket costs for participating in a clinical trial?
No, participants rarely pay anything out of pocket. In fact, you are often compensated for your time.
The trial sponsor (the company running the trial) covers all research-related costs, including the investigational treatment and study-specific tests. Your health insurance typically covers standard care costs you would have anyway.
Most trials also provide reimbursement for travel and other expenses. The informed consent document you sign will clearly outline all financial aspects. Participating in a trial should never create a financial burden.
Predict Dropouts in Real Time with PrivacySafe Dataand Save Your Trial
The world of clinical trial patient support is evolving from basic logistics to a comprehensive, data-driven ecosystem. We are moving beyond guesswork, using real feedback and engagement patterns to design support services that truly work. This mirrors the broader shift toward personalized medicine.
Federated learning is at the heart of this change. Instead of analyzing data in isolated silos, federated platforms allow researchers to find patterns across diverse populations while keeping sensitive patient information secure and private. This enables a deeper understanding of what support strategies work best for different patient groups.
At Lifebit, our platform is built on this vision of secure, collaborative research. Our Trusted Research Environment (TRE) and other components deliver insights that were impossible just years ago. We help researchers access diverse genomic data from global populations, addressing critical imbalances in current research.
The future of clinical trial patient support is an interconnected system where every piece of datafrom wearables to patient-reported outcomesfeeds into a larger understanding of trial success. With real-time insights, support teams can move from being reactive to proactive, identifying and solving problems before they lead to patient dropout.
Learn how Lifebit’s platform powers patient-centric research


