Precision Medicine Meets Machine Learning – A Healthcare Revolution

Why Machine Learning Precision Medicine Is Changing Healthcare Today
Machine learning precision medicine is revolutionizing how we diagnose, treat, and prevent disease by analyzing vast amounts of patient data to deliver personalized care. Here’s what you need to know:
Key Applications:
- Genetic Analysis: AI predicts which genetic variants cause disease with remarkable accuracy.
- Early Detection: Machine learning identifies high-risk patients up to 5 years earlier than traditional methods.
- Treatment Optimization: Algorithms predict which therapies will work best for individual patients.
- Immune System Insights: AI decodes complex immunological data to personalize cancer and autoimmune treatments.
The Impact:
- The global precision medicine market is projected to reach $185.7 billion by 2030.
- Multimodal AI approaches show an average 6.4% increase in predictive accuracy over single-data methods.
- AI models can now analyze electronic health records, genomics, and immunology data together for a complete patient view.
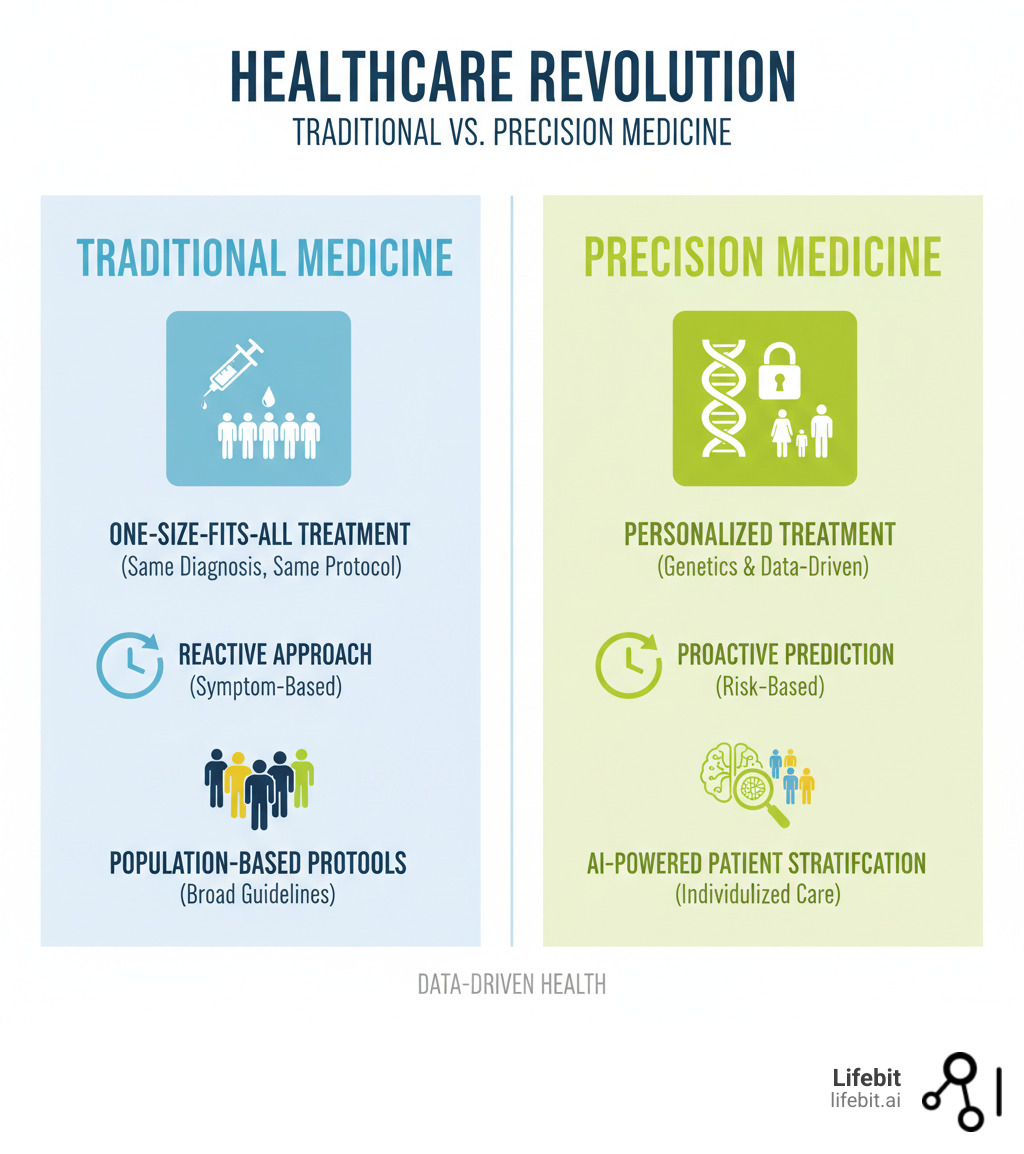
For decades, medicine followed a one-size-fits-all approach, where a patient with diabetes received the same treatment as millions of others, regardless of their unique genetics or lifestyle. That’s changing fast.
Machine learning is enabling a shift from reactive to predictive and personalized healthcare. Instead of waiting for symptoms to worsen, AI algorithms integrate data from electronic health records, genetic sequencing, and immunological tests to predict who will get sick and which treatments will work.
The breakthrough is the ability to analyze millions of data points in seconds, revealing patterns no human could spot. This makes rare genetic variants identifiable and treatment responses predictable.
However, this revolution faces challenges: data silos, privacy concerns, and AI “black boxes” that clinicians struggle to trust. For pharma companies and research institutions, the question isn’t if they should adopt this technology, but how to do it securely, ethically, and at scale.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. We’ve spent over a decade building federated platforms that enable secure, compliant machine learning precision medicine across siloed datasets, drawing on my work in computational biology, AI, and genomics.
Quick Machine learning precision medicine definitions:
The Engine of Findy: How AI Opens up Patient Data
Imagine trying to solve a jigsaw puzzle with millions of pieces scattered across different rooms, each representing a fragment of a patient’s health story: genetic code, immune system patterns, and years of doctor’s notes.
This is the reality of modern healthcare data—and it’s why machine learning precision medicine is so critical.
For decades, doctors made decisions based on limited snapshots of health. Machine learning changes this by integrating data from electronic health records, genetic sequencing, medical imaging, and immunological profiles. This creates a truly holistic view of each patient—not just their symptoms or genes, but everything.
The technology excels at predictive modeling—forecasting disease progression and treatment efficacy. It performs multi-omics analysis, uncovering hidden interactions between genomics, proteomics, and other biological layers. AI was designed to spot patterns across millions of data points that humans simply can’t see.
Studies show that multimodal AI approaches—those combining multiple data types—achieve an average 6.4% increase in predictive accuracy over single-source models. In medicine, where early detection saves lives, every percentage point matters.
The real breakthrough is identifying non-linear relationships that traditional statistics miss. A subtle genetic variant, a specific immune response, and an environmental factor might predict disease risk, but only when analyzed together. This makes data integration the foundation of modern precision medicine.
At Lifebit, our federated platform solves this integration challenge while maintaining security and compliance. Opening up patient data isn’t just a technical problem—it’s a trust, privacy, and collaboration problem.
Opening Genetic Secrets with AI
Your genome contains roughly three billion base pairs. Finding the critical variants that cause disease or determine drug response used to be nearly impossible. Now, machine learning for genomics makes it possible in hours.
The process starts with variant calling, where AI algorithms like DeepVariant accurately spot genetic differences older methods would miss. But finding variants is only half the battle. The harder question is what they mean.
This is where pathogenicity prediction is crucial. AI models like AlphaMissense and PrimateAI-3D analyze variants to predict their clinical significance, reclassifying many previously labeled “unknown significance.” Other tools like SpliceAI predict how variants affect gene splicing, while deep learning approaches identify large-scale copy number variations.
In immunology, MHC-peptide binding predictions have opened new frontiers. AI models like NetMHCpan-4.0 and BigMHC predict how peptides bind to immune molecules, which is essential for developing personalized vaccines and immunotherapies. These computational predictions replace expensive, time-consuming lab experiments.
As a result, genetic risk assessment has evolved from crude statistics to personalized predictions based on an individual’s unique genomic profile. For those interested in diving deeper, the scientific community continues to publish groundbreaking research. Learn more about machine learning applications in genetics and genomics.
Decoding the Immune System for Personalized Therapy
If genomics is complex, immunology is complex and chaotic. Understanding this system well enough to personalize therapy seemed impossible until recently, as techniques like flow cytometry and CyTOF generate datasets too vast for traditional analysis.
Machine learning precision medicine thrives in this environment. The first step is dimensionality reduction, where techniques like UMAP compress complex data into interpretable visualizations. From there, clustering algorithms identify distinct immune cell populations, distinguishing subtle differences that might predict a patient’s response to immunotherapy.
The real magic is predictive modeling. By analyzing immune cells, AI can forecast disease progression and treatment responses. In rheumatoid arthritis, models can predict which patients will respond to expensive anti-TNF-α inhibitors before treatment begins, saving time, money, and suffering.
In autoimmune diagnostics, AI models analyzing immunofluorescence images recognize antinuclear antibody patterns with performance matching human specialists, achieving F1 scores of 0.86. This helps diagnose conditions like lupus, where early detection is critical.
AI also excels at cytokine and chemokine analysis, identifying patterns in these immune messengers that correlate with therapeutic outcomes. This capability helps predict which patients will benefit from checkpoint inhibitors in immuno-oncology and which might experience dangerous side effects.
Changing EHRs into Predictive Health Maps
Electronic Health Records (EHRs) often frustrate clinicians with disorganized information. A typical patient’s EHR contains thousands of data points, but extracting actionable insights is difficult.
Machine learning precision medicine transforms this potential into reality, creating predictive health maps that forecast disease risk and optimal treatment paths.
The process involves several steps:
- Data harmonization to reconcile different coding systems.
- Natural Language Processing (NLP) to extract information from unstructured clinical notes.
- Feature engineering to prepare data for ML models.
- Risk stratification to identify high-risk patients.
- Phenotyping to group patients by clinical characteristics.
- Model validation to ensure reliable predictions.
- Continuous monitoring to maintain accuracy and detect bias.
NLP is especially important, as it can extract symptoms, diagnoses, and treatment responses from physicians’ narrative notes. The results are dramatic: one ML model analyzing EHR data predicted the need for autoimmune disease testing up to 5 years earlier than traditional methods.
Risk stratification helps healthcare systems proactively manage patients at high risk for readmission or adverse reactions. Phenotyping reveals that a single diagnosis can mask different underlying mechanisms, allowing for more targeted treatments.
Throughout this process, maintaining data quality and privacy is paramount. At Lifebit, our federated platform addresses this by enabling analysis across distributed datasets without centralizing sensitive information, ensuring researchers gain insights while patient data remains secure.
Real-World Impact of Machine Learning Precision Medicine
Machine learning precision medicine is already making a measurable difference in how we diagnose diseases, choose treatments, and predict health outcomes.

In hospitals today, AI systems screen for diabetic retinopathy with the accuracy of ophthalmologists, and ML models analyze a patient’s genetic profile to predict which medication will work best before the first dose.
This shift moves medicine from reactive to proactive. Instead of waiting for symptoms to worsen, clinicians can spot disease years earlier and avoid prescribing ineffective or harmful medications.
- Improved diagnostics are not just incrementally better—they’re fundamentally different. By processing genetic, imaging, and clinical data simultaneously, AI identifies patterns impossible for humans to spot, leading to earlier, more accurate diagnoses.
- Treatment optimization reduces the frustrating trial-and-error approach. Predicting responses based on a patient’s unique biology minimizes the time to find effective therapies.
- Reduced adverse events occur when AI flags potential drug interactions or identifies patients at risk for severe side effects based on their genetic markers, preventing harm before it happens.
- Patient stratification at scale makes clinical trials more efficient. AI identifies subgroups of patients who share similar disease trajectories, ensuring therapies reach those most likely to benefit.
What ties this together is the holistic patient view that AI enables. By integrating health records, genetics, and immunology data, we see the complete picture of a patient’s health, making truly personalized, preventive care possible.
Case Study: Machine Learning Breakthroughs in Autoimmune Disease
Autoimmune diseases like rheumatoid arthritis are notoriously complex, making them an ideal testing ground for machine learning precision medicine.
The most striking breakthrough is in early diagnosis. An ML model analyzing EHRs from over 161,000 individuals predicted the need for autoimmune disease testing up to 5 years earlier than traditional methods. That’s five years of potential disease progression that can be prevented or managed. Explore the real-world study on early detection.
For rheumatoid arthritis patients, AI models now predict response to biologic therapies by integrating clinical and genomic data. This allows doctors to choose the right treatment from the start, avoiding months of ineffective therapy.
Clinical decision support tools like the REVAMP platform are emerging to guide treatment by stratifying patients using multi-omics data. In diagnostics, AI models analyzing antinuclear antibody patterns have achieved F1 scores of 0.86, matching the accuracy of experienced specialists and standardizing a traditionally subjective process.
Beyond diagnosis, AI is uncovering new biomarkers by identifying patterns in cytokine and protein profiles, opening doors to new therapeutic approaches.
Oncology: The Vanguard of Precision Medicine
While autoimmune disease represents a burgeoning frontier, oncology has long been the vanguard of precision medicine, with machine learning now accelerating progress at an unprecedented rate. For years, cancer treatment has been moving away from a one-size-fits-all chemotherapy model toward targeted therapies based on the specific molecular drivers of a patient’s tumor.
Machine learning amplifies this in several key ways:
- Genomic-Driven Therapies: AI algorithms are essential for interpreting the massive datasets from next-generation sequencing (NGS) panels. They identify actionable mutations (like EGFR in lung cancer or BRAF in melanoma) and match patients to approved targeted therapies or clinical trials with greater speed and accuracy. This process, known as molecular tumor boarding, is increasingly supported by AI-powered platforms that synthesize genomic data with the latest clinical evidence.
- Computational Pathology: AI is transforming histopathology. Deep learning models can now analyze digital scans of tissue slides (whole-slide images) to perform tasks that were previously impossible or highly time-consuming for human pathologists. For example, models can predict a tumor’s grade, its metastatic potential, and even the presence of specific genetic mutations like microsatellite instability (MSI) directly from the image, guiding immunotherapy decisions in colorectal cancer.
- Predicting Immunotherapy Response: Immuno-oncology, particularly the use of checkpoint inhibitors, has revolutionized cancer care. However, only a subset of patients respond. Machine learning models are being developed to predict who will benefit by integrating data from the tumor microenvironment, the patient’s immune cell profile (from blood tests), and tumor genomics. This helps avoid ineffective treatments and their significant side effects.
Measurable Outcomes and Clinical Benefits
The impact of machine learning precision medicine shows up in the numbers. Identifying high-risk patients for autoimmune disease 5 years earlier means thousands can begin treatment before irreversible damage occurs.
In chronic kidney disease, AI models have identified distinct disease subtypes that respond differently to treatments, enabling more personalized care. Similarly, deep learning models are predicting the conversion to wet age-related macular degeneration with enough accuracy to intervene earlier and preserve vision.
The integration of various data types through AI provides a complete view of the patient. Health records show what’s happened, genetics reveal what’s possible, and immunology data explains what’s happening now. Together, they create a dynamic health map that guides treatment.
Across studies, multimodal AI approaches have demonstrated an average increase of 6.4% in predictive accuracy compared to single-data methods. In healthcare, a 6.4% improvement translates to thousands of better diagnoses, more effective treatments, and prevented complications. These outcomes are not the ceiling—they’re the foundation for what’s to come.
Navigating the Problems: Challenges and Ethics in AI-Powered Medicine
The promise of machine learning precision medicine is extraordinary, but the path to real-world implementation is filled with technical, ethical, and practical barriers.

The biggest obstacles aren’t about whether the technology works—they’re about making it work safely, fairly, and at scale.
- Data silos remain a frustrating barrier, with patient information locked in disconnected systems that don’t communicate.
- Model bias is a serious risk. AI learns from the data we feed it, and if that data reflects historical inequalities, the AI will learn those biases, potentially harming underrepresented groups.
- The black box problem keeps clinicians wary. When an AI model can’t explain why it made a prediction, it’s difficult for a doctor to trust it or explain it to a patient.
- Regulatory approval lags behind research. Few multimodal ML health applications have obtained FDA approval, creating a gap between what’s possible and what’s cleared for clinical use.
Underlying all this are fundamental ethical considerations, such as accountability when an AI makes a mistake and balancing AI’s benefits with patient autonomy. These problems require collaboration between technologists, clinicians, ethicists, and policymakers.
The Data Dilemma: Ensuring Quality and Privacy
Machine learning precision medicine is only as good as its data. Unfortunately, healthcare data is often messy, fragmented, and extremely sensitive.
EHRs are notorious for data quality issues like missing fields and inconsistent coding. Before any analysis, this information must be harmonized into a common language—a tedious but necessary step.
Layered on top are privacy concerns. Health data is deeply personal, and regulations like GDPR and HIPAA exist for good reason. Complying with them while enabling the data sharing AI needs is a major challenge. You can’t just centralize all patient data in one database.
This is where federated learning is a game-changer. Instead of moving data to the AI, you bring the AI to the data. Models can be trained across multiple institutions without the raw data ever leaving its secure location. Each site keeps control while contributing to a shared model.
At Lifebit, our platform is built around this principle. Our Trusted Research Environment (TRE) and Trusted Data Lakehouse (TDL) enable secure collaboration across distributed data. Researchers can work with global biomedical data without compromising privacy or security, following FAIR principles (Findable, Accessible, Interoperable, Reusable) to build scalable solutions.
The Regulatory Maze: From Lab to Clinic
Even a perfect, unbiased, and transparent AI model is useless if it cannot be legally used in clinical practice. Navigating the regulatory landscape is one of the most significant hurdles for machine learning precision medicine. Traditional medical device approval pathways were designed for static hardware or software, not for adaptive AI algorithms that can learn and change over time.
Regulators like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are grappling with how to evaluate these “Software as a Medical Device” (SaMD) products. Key questions include:
- How do you approve a model that continuously evolves? An algorithm that improves its performance with new data presents a challenge for a static approval process.
- What evidence is required? Demonstrating clinical validity for a multimodal AI model that uses complex data inputs is far more complicated than for a traditional diagnostic test.
- How is post-market surveillance handled? Monitoring a model’s performance in the real world to detect performance drift or the emergence of bias is critical.
In response, the FDA has proposed a new framework, outlined in its “AI/ML-Based SaMD Action Plan.” This plan envisions a “predetermined change control plan” where developers can specify planned modifications to their algorithm (e.g., how it will retrain on new data) and have that plan pre-approved. This approach aims to balance the need for rigorous oversight with the dynamic nature of AI, but its implementation is still in early stages, creating a bottleneck between groundbreaking research and widespread clinical adoption.
Building Trust in Machine Learning Precision Medicine
Technology fails when people don’t trust it. For machine learning precision medicine, building trust with clinicians and patients is the real challenge.
Clinicians are rightly skeptical of algorithms that can’t explain their reasoning. Trust is built through consistent performance and transparency. That’s where Explainable AI (XAI) comes in. Instead of just a prediction, XAI systems show their work. Techniques like LIME (Local Interpretable Model-agnostic Explanations) and SHAP (SHapley Additive exPlanations) are becoming critical. LIME explains a single prediction by creating a simpler, understandable model around that specific case, while SHAP uses principles from game theory to assign an importance value to each feature (like a specific gene or lab result), showing which factors “pushed” the model’s prediction higher or lower. This allows a doctor to see why the AI flagged a patient as high-risk, integrating the model’s insights with their own clinical judgment.
Model validation must be continuous. An AI that works in one hospital might fail in another, and performance can drift over time. We need clear protocols for testing AI in diverse settings and tracking its performance to catch problems before they harm patients.
Algorithmic fairness is a matter of justice. If an AI model works better for some demographic groups than others, it perpetuates inequality. Detecting and mitigating these biases requires constant vigilance, from examining training data for representativeness to testing models across diverse populations.
We also need clear frameworks for accountability. When an AI makes a wrong prediction, who is responsible? The developer? The hospital? The clinician? These questions need answers before AI becomes more deeply embedded in healthcare.
At Lifebit, we’ve designed our federated governance capabilities with these concerns front and center. Security, compliance, and transparency are built into our foundation, because the most sophisticated AI is worthless if people don’t trust it.
The Future is Now: What’s Next for AI in Patient Care?
We are at the threshold of a healthcare revolution. The promise of machine learning precision medicine isn’t just about better treatments tomorrow—it’s about reshaping how we think about health today.
The vision is clear: healthcare that is predictive, preventive, personalized, and participatory (P4 medicine). AI is the engine making this possible.
- Proactive healthcare is becoming a reality. AI systems will analyze data from wearables, genetic profiles, and health records to spot trouble months before symptoms appear.
- Digital twins, virtual replicas of an individual’s biology, are moving from science fiction to fact. Doctors could test medications on a digital twin to predict a patient’s response, revolutionizing drug development and treatment planning.
- AI in drug findy is already cutting years off development timelines by identifying promising drug candidates far faster than traditional methods.
- Large Language Models are changing how medical information flows, summarizing clinical notes and synthesizing research to amplify human expertise.
The real breakthrough will come from sophisticated multi-modal data fusion, weaving together genomic, proteomic, clinical, and other data to reveal a complete picture of a patient’s health.
The global precision medicine market, valued at $69.7 billion in 2022, is projected to surge to $185.7 billion by 2030. This growth reflects real demand for healthcare that is built for you.
However, this potential is meaningless if data remains locked in silos. The future of machine learning precision medicine depends on secure, collaborative platforms that let institutions share insights without compromising privacy.
This is where Lifebit’s federated AI platform comes in. We provide the infrastructure that makes this future possible today, enabling secure, real-time access to global biomedical data while keeping sensitive information within each institution’s control. With built-in harmonization, advanced AI/ML analytics, and federated governance, we help organizations open up previously unreachable insights.
Our Trusted Research Environment (TRE) provides a secure space for collaborative analysis, while our Trusted Data Lakehouse (TDL) and R.E.A.L. (Real-time Evidence & Analytics Layer) deliver immediate, AI-driven insights. We are building trust—trust that data can be shared securely, that privacy will be protected, and that the promise of AI in medicine will benefit everyone.
The future of patient care is already here. Every day, machine learning precision medicine moves from possibility to practice, and we are committed to ensuring that change happens securely, ethically, and at the speed patients deserve.
Discover how to accelerate your research with federated data analysis

