Detailed Guide to Clinical Research SaaS Tech Stacks

Why Modern Clinical Trials Are Failing—And How Technology Can Fix It
Clinical Research SaaS: The Tech Stack Behind Modern Trials is the foundation for overcoming the industry’s biggest challenges: astronomical costs, decade-long timelines, and low success rates.
The modern tech stack consists of four integrated layers:
- Infrastructure Layer – Cloud platforms (AWS, Azure, GCP) providing scalable, GxP-compliant compute and storage, freeing up to 30% of R&D IT spending.
- Data Layer – Data lakes and warehouses enabling interoperability across siloed systems.
- Application Layer – Core systems like CTMS, EDC, and IRT/RTSM that streamline trial execution.
- Analytics Layer – AI/ML platforms turning raw data into actionable insights, with GenAI projected to open up $13-25 billion in annual value.
The stakes are high. With timelines exceeding a decade, costs surpassing $2 billion per asset, and barely 13% of drugs making it to launch, legacy systems are failing. The shift to a modern SaaS-driven infrastructure is no longer optional—it’s the difference between real-time insights and data sitting in silos for months, and between automated workflows and budget-draining manual processes.
As CEO and Co-founder of Lifebit, I’ve spent over 15 years building the federated platforms that define modern Clinical Research SaaS. This guide draws from our experience powering data-driven drug findy for global pharma and public sector institutions to show you what’s working in 2025.
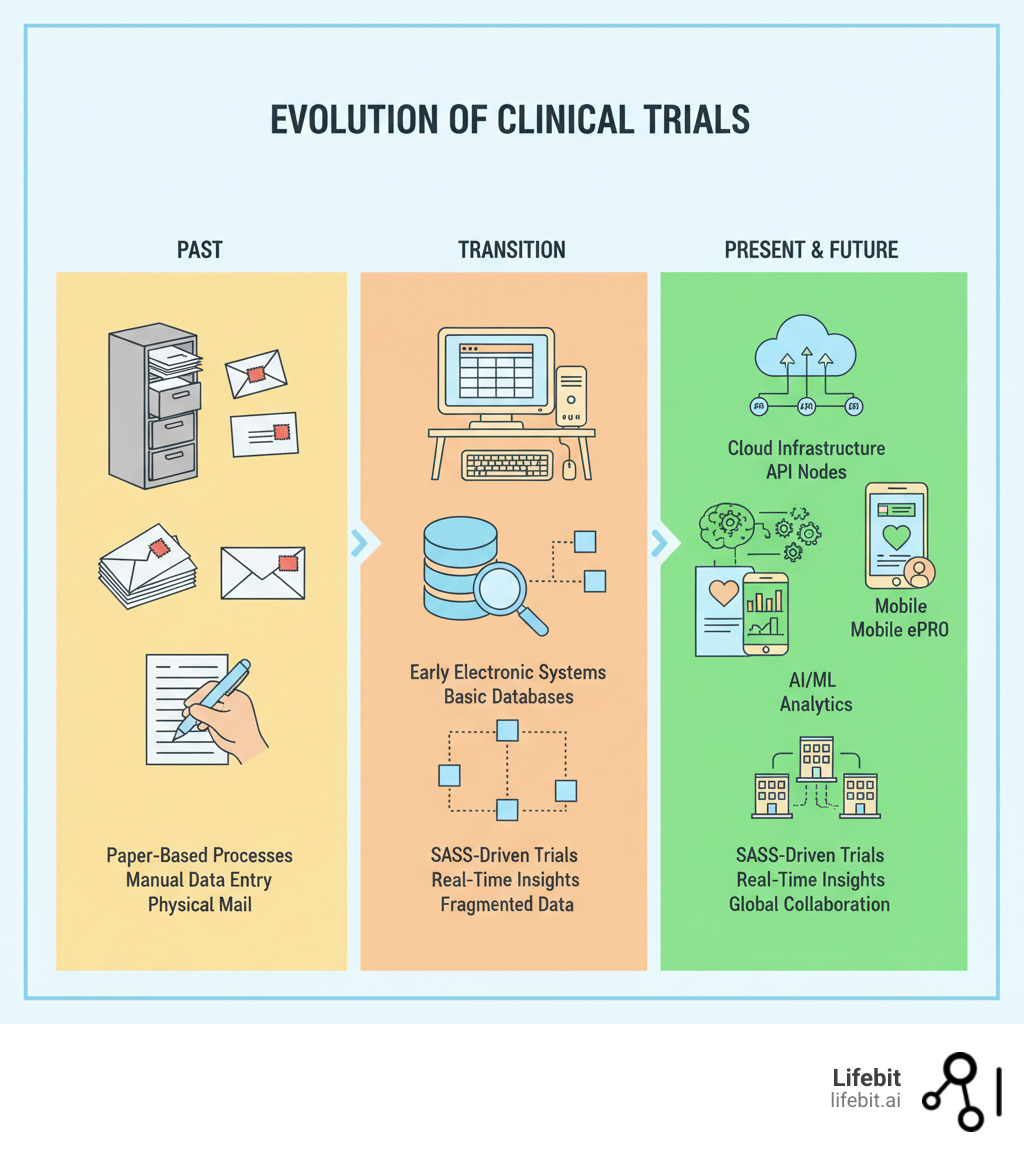
Basic Clinical Research SaaS: The Tech Stack Behind Modern Trials terms:
- current systems and technology in clinical trials
- interactive voice response system clinical trials
- remote monitoring in clinical trials
The Anatomy of a Modern Clinical Trial Tech Stack: The Four Core Layers
A functional clinical trial platform is an integrated ecosystem, not just a collection of siloed technologies. It allows data to flow, systems to communicate, and teams to focus on science. This ecosystem is built on four essential layers.
Layer 1: The Infrastructure (Cloud & Scalability)
Infrastructure is the bedrock, and in 2025, that means the cloud. On-premise data centers are a capital-intensive bottleneck, while cloud platforms like AWS, Azure, and GCP offer the elastic, on-demand compute and storage modern trials demand. By moving to the cloud, organizations are freeing up to 30% of R&D IT spending, reallocating funds from hardware maintenance to scientific innovation. The COVID-19 vaccine trials demonstrated this power, with sponsors leveraging massive cloud compute capabilities to analyze genomic data and run simulations at a scale and speed previously unimaginable.
A hybrid cloud model is a common approach, balancing the control of on-premise systems with the power of the public cloud for intensive tasks like AI model training. GxP compliance is non-negotiable. Major cloud providers now offer validated blueprints and dedicated services for GxP environments, ensuring regulatory standards are met through automated controls and audit trails. This is often managed via Infrastructure as Code (IaC), where tools like Terraform define and manage infrastructure, ensuring that validated environments can be reproduced perfectly and consistently.
Behind the scenes, containerization (Docker) and microservices architecture are key. Instead of a single, monolithic application where one failure can bring down the entire system, functionality is broken into smaller, independent services (e.g., a service for patient registration, another for data validation). Kubernetes orchestrates these containers, automating deployment and scaling. The result is a highly resilient platform where deployment times drop from hours to minutes, and a failure in one service doesn’t impact others, dramatically improving uptime.
Layer 2: The Data (Interoperability & a Single Source of Truth)
Data is the lifeblood of modern trials, but its sheer volume—projected to exceed 2,314 exabytes in healthcare—is a challenge. Modern platforms often use a data lakehouse architecture, combining the flexibility of a data lake (for storing raw, unstructured data like genomic sequences for exploratory analytics) with the performance and governance of a data warehouse (for refined, structured data used in reporting).
The biggest hurdle is interoperability. Systems often don’t speak the same language. FHIR (Fast Healthcare Interoperability Resources) provides standardized APIs and data formats to act as a universal translator for healthcare data. Equally important is metadata management, which documents the “data about the data”—its origin, format, and meaning, often managed in a data catalog. This entire approach aligns with the FAIR data principles:
- Findable: Data is assigned a unique and persistent identifier and described with rich metadata.
- Accessible: Data can be retrieved using a standardized, open communication protocol.
- Interoperable: Data uses a formal, shared language and vocabulary so it can be combined with other data sources.
- Reusable: Data is well-described with a clear usage license, allowing it to be used for future studies.
At Lifebit, our platform is built on these principles, enabling secure, federated analysis of global biomedical data without moving sensitive information, solving a core challenge of multi-institutional research.
Layer 3: The Applications (Streamlining Trial Execution)
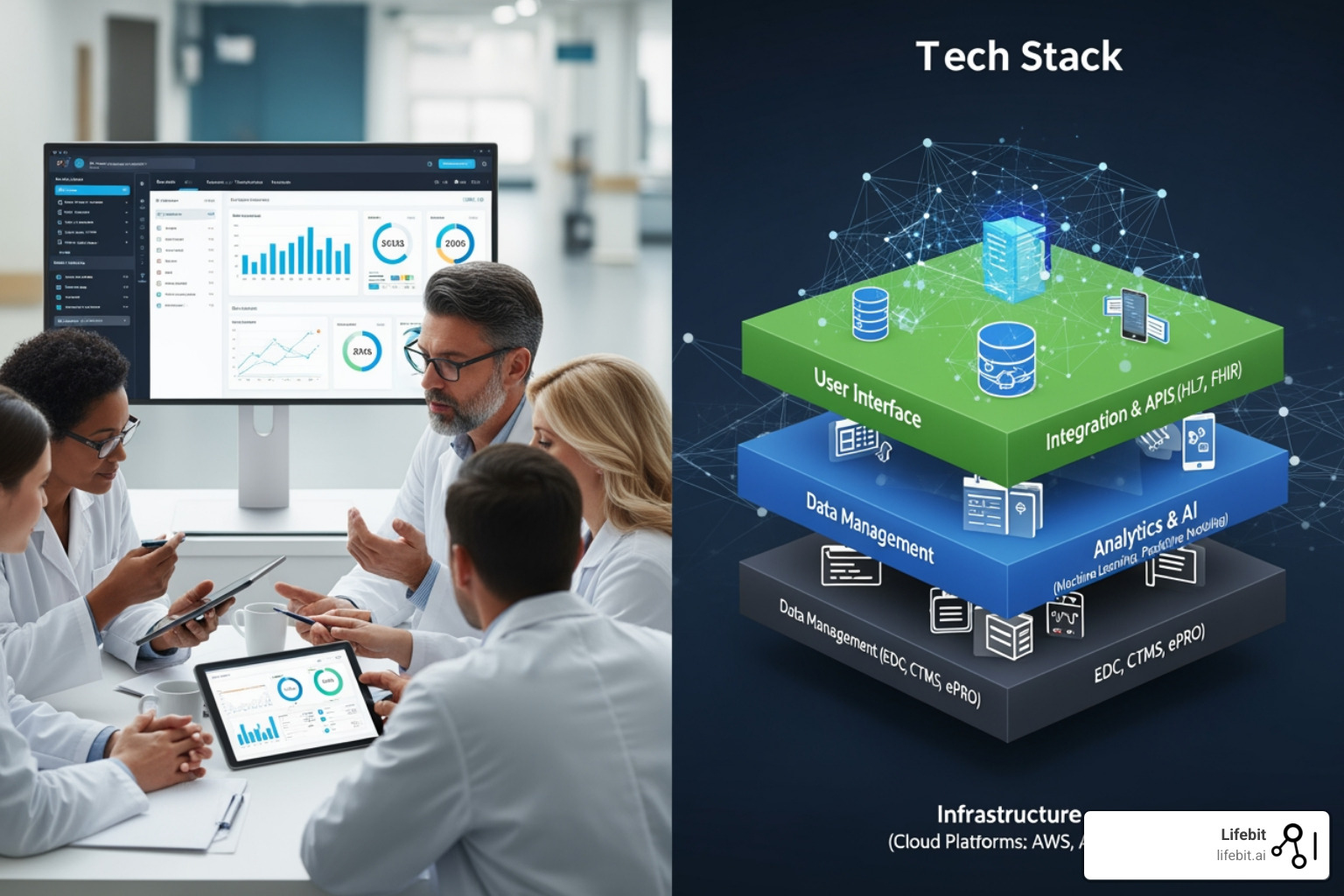
The application layer includes the tools teams use daily to execute trials.
- Clinical Trial Management Systems (CTMS) are the operational backbone, managing planning, site selection, and monitoring. A modern CTMS integrates with other systems to provide a holistic view of trial progress, including site management, financial tracking, and monitoring.
- Electronic Data Capture (EDC) systems have replaced paper, enabling remote monitoring and real-time error checking. This can cut the time to database lock by more than 50%. Modern EDCs are moving beyond simple eCRFs to support direct data capture from medical devices and EHRs.
- ePRO/eCOA (Electronic Patient-Reported Outcomes/Clinical Outcome Assessments) empower patients to report experiences in real-time via mobile devices, improving data quality and reducing burden. This area is expanding to include data from wearables and sensors, creating a continuous stream of objective data.
- IRT/RTSM (Interactive Response Technology/Randomization & Trial Supply Management) platforms have evolved into sophisticated logistics engines. This $1.2 billion market (growing at 14% CAGR) automates complex randomization schemes and manages global drug supply chains, preventing stockouts and minimizing waste.
- electronic Trial Master File (eTMF) systems are the digital repository for all essential trial documents (e.g., protocols, consent forms). An eTMF is critical for maintaining an audit-ready state and ensuring compliance with regulations like ICH-GCP.
- Social media for patient recruitment leverages platforms like Facebook to find and engage diverse patient populations, expanding the pool of participants beyond traditional methods.
Layer 4: The Analytics (From Data to Decisions)
The analytics layer transforms raw data into actionable intelligence.
- AI and Machine Learning are now essential tools for predicting molecular properties, optimizing trial designs, and identifying at-risk patients. This includes identifying digital biomarkers—data from digital devices like smartphones or wearables—that can serve as objective indicators of disease progression.
- Predictive analytics uses historical and real-time data to forecast outcomes, such as patient enrollment rates, allowing teams to act proactively to prevent delays.
- Real-world evidence (RWE) from sources like EHRs, insurance claims data, and patient registries complements trial data. It provides crucial insights into how drugs perform in real-world clinical practice, supporting label expansion and post-market surveillance.
- Data visualization dashboards make complex data digestible, providing real-time visibility into trial performance and guiding decisions.
A key principle is creating reusable data products for AI. Instead of ad-hoc data prep, this involves building curated, standardized datasets to consistently feed ML models, accelerating the entire analytics cycle. Lifebit’s Trusted Research Environment (TRE) embodies this, providing a secure space for researchers to deploy AI models on harmonized, research-ready data.
How AI and Automation are Revolutionizing Clinical Research SaaS: The Tech Stack Behind Modern Trials
In 2025, AI and automation are no longer optional add-ons; they are core components of Clinical Research SaaS: The Tech Stack Behind Modern Trials that drive efficiency, quality, and speed. They augment human intelligence, freeing scientists from tedious work to focus on innovation.
Automating Core Processes for Unprecedented Efficiency
- Metadata-driven automation uses standardized definitions (e.g., from CDISC) to automatically generate case report forms (CRFs), validation checks, and statistical analysis plans, ensuring consistency from study start.
- Automated CRF generation can reduce the time to build and launch a study to under 24 hours, while automated CSR generation assembles data, tables, and narratives to shrink submission timelines.
- AI-powered data cleaning uses anomaly detection algorithms to flag outliers and inconsistencies in real-time, accelerating data cleaning cycles by up to 50% and leading to faster database lock.
- AI-driven risk-based monitoring (RBM) analyzes data from multiple sources to identify high-risk sites and data patterns. This allows sponsors to focus monitoring resources where they are most needed, reducing on-site monitoring days by up to 33% while improving data quality.
- Automated Safety Case Processing: AI can automate the intake and processing of adverse event (AE) reports, extracting relevant information from unstructured text, coding it using MedDRA, and flagging serious events for immediate review. This can reduce manual effort by over 70% and ensures faster regulatory reporting.
- Intelligent Source Data Verification (SDV): Instead of manually verifying 100% of data points, AI can perform targeted SDV by predicting which data points are most likely to contain errors or have the highest impact on trial outcomes, maintaining quality while reducing the burden on monitors.
The Impact of Generative AI on the Clinical Development Lifecycle
Generative AI represents a massive leap in capability, moving from analyzing existing data to creating novel content. McKinsey projects GenAI will create $13 billion to $25 billion in annual value for pharma clinical development.
- GenAI for protocol optimization: A poorly designed protocol is a primary cause of trial failure. GenAI can analyze vast libraries of past trial protocols and real-world data to suggest optimal trial designs. For example, it might identify inclusion/exclusion criteria that are unnecessarily restrictive and hinder recruitment, suggesting alternatives that maintain scientific rigor while improving feasibility.
- AI for patient cohort selection: AI algorithms analyze multi-dimensional patient data—including genomics, EHRs, and imaging—to identify the precise patient cohorts most likely to respond to a treatment. This increases the probability of trial success and helps fulfill regulatory demands for improved trial diversity.
- Synthetic Data Generation: In rare diseases where patient data is scarce, GenAI can create statistically realistic “synthetic” patient data. This data can be used to augment training sets for ML models or test analytical hypotheses without compromising patient privacy.
- Automated Content Creation: GenAI can rapidly generate drafts of essential documents, such as patient-facing informed consent forms written in plain language or clinical study report summaries. This frees up medical writers to focus on refinement rather than starting from a blank page.
Challenges like model validation (“hallucinations”), evolving regulations, and organizational inertia are real. McKinsey analysis on GenAI in pharma However, the convergence of AI with a modern Clinical Research SaaS: The Tech Stack Behind Modern Trials creates opportunities we cannot afford to ignore. Lifebit’s federated AI platform integrates these capabilities securely, enabling researchers to deploy validated AI models on harmonized data without compromising patient privacy.
Strategic Blueprint: How to Design and Modernize Your R&D Tech Stack
Modernizing your tech stack is a strategic decision, not just a software swap. A well-designed architecture provides a competitive advantage, while a poor one leads to wasted time and money.
Core Principles for a Flexible and Scalable Architecture
A future-proof platform is built on these fundamental principles:
- API-first design: Build applications assuming they will connect with other systems, creating a flexible connectivity layer for adding new capabilities. Lifebit’s platform is built on this principle for seamless interoperability.
- Centralized data exchange: Create governed pathways for information to flow where it’s needed, breaking down data silos without forcing all data into a single database.
- Modularity: Use microservices to break systems into independent modules, allowing you to update or replace components without disrupting the entire stack.
- Interoperability: Ensure data can be understood across systems using standards like FHIR and robust metadata management.
- Future-proofing: Build an extensible architecture that can integrate new technologies as they emerge and adapt to evolving regulations.
- Avoiding tech debt: Invest in well-architected systems and continuous refactoring to prevent the long-term costs of quick, easy solutions.
Choosing Your Modernization Strategy: Platform, Best-of-Breed, or Hybrid?
You have three main approaches to modernization:
- The platform approach involves adopting an integrated suite from a single vendor. This simplifies integration but can lead to vendor lock-in and less flexibility.
- The best-of-breed approach means selecting the top solution for each function. This offers cutting-edge tools but creates significant integration and management overhead.
- The hybrid model offers a pragmatic balance. It uses a core platform for foundational functions while integrating best-of-breed tools for specialized needs. This is the most common strategy, but it requires a robust API and data strategy to succeed. Lifebit’s federated platform is designed for this hybrid reality, providing core infrastructure that integrates with your existing tools.
| Strategy | Cost Implications | Flexibility & Customization | Integration Complexity |
|---|---|---|---|
| Platform | Lower initial integration costs, potential for higher long-term licensing | Limited customization, dependent on vendor roadmap | Low to moderate (within platform), high (external systems) |
| Best-of-Breed | Higher initial integration costs, potentially lower specialized licensing | High flexibility, choose best tools for specific needs | High (across many disparate systems) |
| Hybrid | Moderate initial and ongoing costs | Moderate to high, balance core platform with specialized tools | Moderate (focused integration points) |
The right strategy depends on your organization’s size, complexity, and goals. Choose deliberately based on your long-term strategy, not short-term convenience.
A Lifecycle View: Essential Software for Every Stage of Pharma
The pharmaceutical lifecycle extends far beyond clinical trials, from initial discovery to patient delivery. A comprehensive Clinical Research SaaS: The Tech Stack Behind Modern Trials is part of a larger ecosystem of software that supports every stage with specialized, integrated tools.
Early R&D and Drug Findy
This stage is increasingly driven by intelligent software that accelerates the identification and validation of new therapeutic candidates.
- Computational tools and AI-driven drug design use in silico screening to model molecular interactions and predict a compound’s efficacy and toxicity, reducing the need for expensive wet lab experiments. AI platforms like DeepMind’s AlphaFold, which predicts protein structures, are revolutionizing target identification.
- Bioinformatics Platforms: These platforms are essential for analyzing the massive datasets generated by genomics and proteomics, helping researchers identify novel drug targets and discover predictive biomarkers.
- Laboratory Information Management Systems (LIMS) are the lab’s operational backbone, managing the lifecycle of samples and tracking workflows to ensure data integrity and chain of custody.
- Electronic Lab Notebooks (ELN) replace paper notebooks, allowing researchers to record experiments in a secure, searchable, and shareable digital format, which facilitates collaboration and protects intellectual property.
Clinical Trials and Regulatory Compliance
This is the core of the Clinical Research SaaS: The Tech Stack Behind Modern Trials, orchestrating human testing and regulatory oversight.
- CTMS and EDC systems, as discussed earlier, manage trial operations and patient data collection.
- Electronic Quality Management Systems (eQMS) are vital for maintaining GxP standards. An eQMS is a unified platform for managing quality processes, including document control, employee training records, audits, and Corrective and Preventive Actions (CAPAs), ensuring a constant state of inspection readiness.
- Regulatory Information Management (RIM) systems act as a command center for managing the complex documentation required for global regulatory submissions, tracking correspondence with health authorities like the FDA and EMA and assembling submission dossiers.
- eTMF (electronic Trial Master File) is the legally required collection of all essential documents that demonstrate the trial was conducted in compliance with GCP guidelines. A dedicated eTMF system ensures these documents are complete, timely, and accessible for audits.
Manufacturing and Supply Chain
Once a drug is approved, the challenge shifts to producing it at scale and delivering it safely to patients.
- Enterprise Resource Planning (ERP) systems integrate data across the business, from finance and procurement to manufacturing and inventory, for a holistic operational view.
- Manufacturing Execution Systems (MES) control and monitor processes on the factory floor, enforcing workflows and creating the electronic batch record (EBR) to ensure product quality.
- Supply Chain Management (SCM) software oversees the complex journey from factory to patient. This includes managing logistics and implementing security features like serialization (track-and-trace). Regulations like the U.S. Drug Supply Chain Security Act (DSCSA) mandate unique identifiers on each drug package to prevent counterfeiting. Some companies are exploring blockchain for an immutable supply chain ledger.
- IoT integration is transforming this stage. Sensors on manufacturing equipment enable predictive maintenance, while smart packaging with embedded sensors can monitor the temperature of sensitive biologics during shipment, ensuring product integrity throughout the cold chain.
Frequently Asked Questions about Clinical Research SaaS
How does a modern SaaS stack ensure data security and compliance?
A modern Clinical Research SaaS: The Tech Stack Behind Modern Trials builds security and compliance in from the ground up.
- Cloud Security: Providers like AWS and Azure offer robust services, including Identity and Access Management (IAM) for granular user controls, end-to-end encryption, and secure networking.
- Compliance Certifications: Reputable SaaS providers demonstrate adherence to global standards like GxP, HIPAA, and GDPR. They provide audit trails, 21 CFR Part 11 compliant e-signatures, and validation documentation for regulators.
- Automated Validation: Modern DevOps practices and Infrastructure as Code (IaC) ensure environments remain consistent and validated. Automated tools monitor systems for security anomalies in real-time.
- Data Governance: Strong governance frameworks ensure data quality and adherence to access policies. Federated models, like those used by Lifebit, allow analysis to be performed on data at its source, which is critical for collaborations where data sovereignty is paramount.
What is the biggest challenge when migrating from a legacy system?
The biggest challenge is not technical; it’s human. Organizational inertia is the primary obstacle to modernization. The risk-averse nature of the clinical trial industry, coupled with a fear of disrupting ongoing trials or failing audits, creates powerful resistance to change.
This is amplified by technical challenges:
- Data silos and integration: Extracting, cleaning, and migrating decades of fragmented data from isolated legacy systems is a monumental task that requires maintaining perfect data integrity.
- Validation overhead: Every new system in a regulated environment requires extensive and resource-intensive validation, which can deter organizations from upgrading.
- Skill gaps: Adopting new cloud, microservices, and AI technologies requires new skill sets that may not be readily available internally.
Overcoming these challenges requires a clear strategy, executive buy-in, and a phased approach that focuses on high-impact areas first to build momentum.
How can we measure the ROI of investing in a new clinical tech stack?
The ROI of a modern Clinical Research SaaS: The Tech Stack Behind Modern Trials goes beyond simple cost savings.
- Reduced cycle times: Measure the time saved in key processes like study start-up, patient recruitment, and database lock. Modern platforms can cut build cycle times by 50%.
- Cost savings: Migrating to the cloud can free up to 30% of R&D IT spending. Automation reduces manual effort, and optimized supply management minimizes drug wastage.
- Increased probability of success: This is where the real value lies. Using AI for better trial design and patient selection can improve success rates. Given that only 13% of drugs in Phase 1 reach launch, even small improvements have a massive financial impact. GenAI alone is projected to create $13 billion to $25 billion in annual value.
- Improved data quality: Better data from automated cleaning and integration leads to fewer queries (up to an 86% reduction) and more reliable go/no-go decisions.
- Faster time to market: Accelerating the R&D pipeline brings therapies to patients sooner, enabling earlier revenue generation and a significant competitive advantage.
The ROI isn’t just what you save—it’s what you gain in speed, quality, and competitive positioning.
Conclusion: Building the Future of Clinical Trials, Today
The path forward is clear. A modern Clinical Research SaaS: The Tech Stack Behind Modern Trials is not an incremental improvement but a complete reimagining of drug development. It’s an integrated ecosystem where data flows freely, intelligence is embedded everywhere, and efficiency is the norm.
The business case is undeniable. Organizations adopting modern SaaS stacks see increased R&D productivity, IT cost reductions of up to 30%, and dramatically shorter timelines. Most importantly, they increase their probability of success in an industry where barely 13% of assets make it to launch, and the average cost per asset exceeds $2 billion.
Technology is the key to faster, smarter drug development. The shift from manual, fragmented processes to automated, intelligent workflows is a survival imperative. With GenAI projected to create $13-$25 billion in annual value, delaying modernization means leaving billions on the table while patients wait.
At Lifebit, our federated AI platform is built for this new era. Our solutions enable secure, real-time access to global biomedical data, allowing large-scale studies and advanced analytics without compromising privacy or compliance.
The future of clinical trials is already here. The technology exists, the frameworks are proven, and the ROI is compelling. Every day spent on legacy systems is a day of missed opportunities.
Find how to build your next-gen R&D tech stack and join the organizations that are already changing clinical research. Because the patients waiting for tomorrow’s treatments can’t afford yesterday’s technology.

