In-Depth Guide to Real-Time Evidence Generation

FDA Approves 85% of RWE Submissions—Get Answers in Weeks, Not Years
Real-time evidence generation is revolutionizing healthcare by analyzing patient data as it’s collected, not years later. While traditional clinical trials take over a decade and cost billions, this approach uses data from everyday medical practice to answer critical questions about drug safety and effectiveness in just weeks or months.
What you need to know:
- Real-World Data (RWD) is raw health information from sources like electronic health records (EHRs), insurance claims, and wearables.
- Real-World Evidence (RWE) is the clinical insight generated from analyzing RWD with rigorous methods.
- Real-time generation means continuously collecting and analyzing this data for faster decisions, better care, and more efficient drug development.
- Regulatory acceptance is growing: the FDA approved 85% of submissions backed by RWE between 2019 and 2021.
- A massive opportunity exists, as only 20% of pharma companies have integrated evidence plans across their product lifecycle.
The shift is urgent. Traditional randomized controlled trials (RCTs) often exclude the elderly, patients with multiple conditions, and diverse populations. Real-time evidence generation reveals how treatments work for everyone in actual clinical settings.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. We’ve spent over a decade building federated data platforms that enable real-time evidence generation for global pharma, public sector agencies, and regulatory bodies. Our work empowers organizations to analyze diverse datasets —from EHRs to genomics —without moving data, accelerating real-time pharmacovigilance and AI-powered evidence generation at scale.
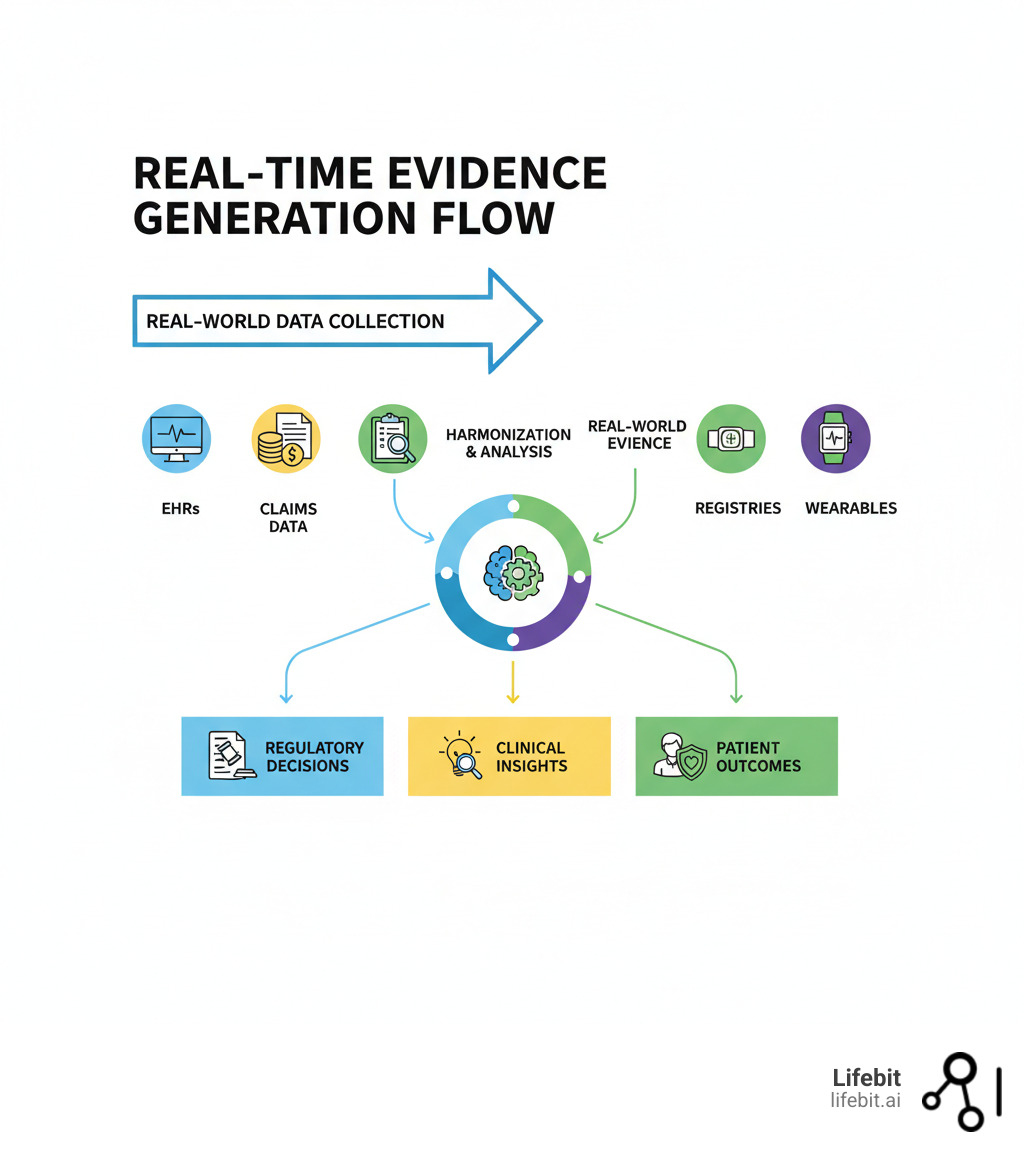
Real-time evidence generation terms explained:
RWD vs RWE in 5 Minutes: The Data Sources That Prove What Works
To master real-time evidence generation, it’s crucial to understand its core components: Real-World Data (RWD) and Real-World Evidence (RWE).
Real-World Data (RWD) is the raw information on patient health collected from everyday sources. Real-World Evidence (RWE) is the clinical insight produced after RWD is analyzed using rigorous scientific methods. In short, RWD is the input, and RWE is the output. For a deeper dive, see our guide: Real-World Data vs. Real-World Evidence: The Ultimate Guide.
For decades, Randomized Controlled Trials (RCTs) have been the gold standard for proving a treatment can work under ideal conditions. However, these perfect conditions don’t reflect real life, as RCTs often exclude patients with multiple health issues, the elderly, or those from diverse demographic backgrounds. This creates an “efficacy-effectiveness gap” where a drug’s performance in a trial doesn’t match its performance in the community.
This is where RWE excels. It captures what happens in routine clinical practice, offering greater generalizability by including patients often left out of trials. RWE reveals insights into diverse populations and tracks long-term outcomes far beyond a typical trial’s follow-up period. For example, an RWE study on a type 2 diabetes treatment revealed higher long-term adherence compared to other options—an insight only visible by observing thousands of patients over time.
Here’s how RCTs and RWE stack up:
| Feature | Randomized Controlled Trials (RCTs) | Real-World Evidence (RWE) |
|---|---|---|
| Primary Goal | Establish efficacy under ideal, controlled conditions | Assess effectiveness and safety in routine clinical practice |
| Patient Population | Highly selected, often homogenous | Diverse, representative of real-world patients |
| Study Environment | Controlled, often academic centers | Routine healthcare settings (hospitals, clinics, pharmacies) |
| Intervention Delivery | Standardized, strict protocol adherence | Variable, reflecting actual clinical practice |
| Outcomes Measured | Efficacy (does it work?), safety | Effectiveness (does it work in practice?), safety, adherence, QoL |
| Bias Control | High (randomization, blinding) | Lower, requires advanced statistical methods to mitigate |
| Generalizability | Limited to study population | High, reflects broad patient experience |
| Cost & Time | High cost, long duration | Lower cost, faster generation, especially for real-time evidence generation |
Real-World Data comes from a wide array of sources:
- Electronic Health Records (EHRs): Digital patient charts containing structured data (diagnoses, lab results) and unstructured data (clinical notes). Unstructured text often holds rich details on disease severity and patient history.
- Insurance claims data: Records of diagnoses, procedures, and treatments created from billing. While powerful for longitudinal analysis of healthcare utilization, they often lack clinical granularity like lab values.
- Disease registries: Organized systems collecting deep, disease-specific data on conditions, such as the European Cystic Fibrosis Society (ECFS) Registry, providing high-quality data for specific research questions.
- Patient-generated data: Information from mobile health apps, wearables (e.g., Apple Watch, Fitbit), continuous glucose monitors, and patient-reported outcome (PRO) surveys that capture daily life experiences and quality-of-life metrics.
- Pharmacy data: Prescription records that reveal medication adherence, switching patterns, and co-medications, which are critical for understanding treatment effectiveness in practice.
- Genomic and Biomarker Data: Data from biobanks and genomic testing that links molecular information to clinical outcomes, forming the bedrock of personalized medicine research.
When combined, these sources provide a 360-degree view of how treatments perform in the real world, forming the foundation of real-time evidence generation. Learn more in our guide on Real-World Data Examples.
Build a Real‑Time RWE Engine: OMOP, NLP, Federated AI—No Data Moves
Changing RWD into RWE requires a robust engine that can continuously ingest, harmonize, and analyze vast, disparate datasets. This is the core of the real-time evidence generation paradigm.

At Lifebit, our federated AI platform is designed for this purpose, enabling secure analysis by working with data where it resides. The engine involves several key stages:
- Data Ingestion: Bringing data from diverse RWD sources into a flexible, scalable system.
- Data Harmonization: Standardizing disparate data into a common data model (CDM). A CDM like OMOP acts as a “universal translator,” mapping different terminologies and coding systems from various sources into a single, consistent format. This makes data interoperable and enables large-scale network studies across multiple institutions or countries.
- Advanced Analytics: Applying complex statistical modeling and pattern recognition to extract meaningful clinical insights.
- AI and Machine Learning (AI/ML): Using algorithms to process data at high speed, identifying trends and anomalies that would otherwise be hidden.
- Natural Language Processing (NLP): Extracting valuable information from unstructured text like clinical notes and physician observations. NLP can unlock critical details not found in structured fields, such as smoking status, disease severity, social determinants of health, and reasons for treatment non-adherence.
- Federated Learning: Training AI models on decentralized datasets without moving sensitive data. In this model, the analytical code is sent to the data’s location, and only aggregated, non-identifiable results are returned. This addresses privacy concerns while enabling collaborative research on a global scale. Our platform uses federated governance to ensure security and compliance.
This technological backbone enables a dynamic, continuous evidence generation process. Learn more about its impact on drug safety in our guide to Real-Time Pharmacovigilance.
The Process of Real-Time Evidence Generation
Generating credible RWE follows a structured process:
- Formulate Research Question: Start with a clear, well-defined clinical or regulatory question.
- Study Design: Choose an appropriate observational design (e.g., cohort, case-control) to minimize bias.
- Protocol Development: Outline the study’s objectives, methods, and analytical plan for transparency, following guidance like the FDA’s framework for RWE.
- Data Analysis: Apply advanced statistical and AI/ML methods to the harmonized data.
- Evidence Synthesis & Reporting: Interpret the results in context and share them transparently through publications or regulatory submissions.
Common Study Designs Used
RWD allows for flexible study designs to capture real-world complexities:
- Observational Studies: These are the most common designs, including cohort studies (following groups over time), case-control studies (comparing groups with and without an outcome), and cross-sectional studies (observing a population at one point in time).
- Pragmatic Clinical Trials: These trials evaluate interventions under usual care conditions, bridging the gap between RCTs and observational studies.
- Retrospective and Prospective Studies: Analyze historical RWD or collect new RWD moving forward, respectively.
- External Control Arms: Use historical RWD to create a comparator group for a single-arm trial. This is especially useful in rare diseases where recruiting a concurrent placebo group is often unethical or infeasible. The FDA has accepted this approach for certain drug approvals.
Choosing the right design is critical for generating credible RWE. For more on this topic, see our article on Real-World Data Clinical Trials.
Unlock Value Fast: Cut Recruitment, Spot Safety Signals in Days, Win Access
Real-time evidence generation is a strategic shift that creates tangible value at every stage of the product lifecycle, from early discovery to post-market monitoring. It enables continuous learning, making drug development faster, smarter, and more cost-effective.
Clinical Trial Optimization
RWD helps design better trials by enabling data-driven feasibility assessments to identify geographic hotspots of eligible patients and optimizing protocols by modeling the impact of different inclusion/exclusion criteria on the potential recruitment pool. This dramatically reduces recruitment times. It also helps refine inclusion/exclusion criteria and identify optimal study sites. In some cases, RWD can be used to create synthetic or external control arms, reducing the need for placebo groups and accelerating trial timelines.
Post-Market Surveillance
Instead of relying on slow, passive surveillance systems that depend on voluntary adverse event reporting, real-time analysis of RWD allows for proactive, active safety monitoring. The FDA’s Sentinel Initiative is a prime example, using a distributed network of healthcare data to actively monitor medical product safety as it’s being used. Our platform supports Real-Time Adverse Drug Reaction Surveillance, enabling organizations to detect safety signals as they emerge.
Market Access and Reimbursement
RWE is now the currency of negotiation with payers. Health Economics and Outcomes Research (HEOR) powered by RWE demonstrates a product’s real-world impact on outcomes, quality of life, and costs. This evidence is crucial for securing reimbursement and negotiating value-based agreements. For example, a manufacturer and payer might agree that a new, expensive drug will be fully reimbursed only if RWE shows it reduces hospital readmission rates by a pre-specified amount within two years, aligning payment with proven patient outcomes.
Regulatory Submissions
The role of RWE in regulatory decisions is growing rapidly. Between 2019 and 2021, the FDA approved 85% of submissions that included real-world evidence. RWE is used for label expansions, new indications, and post-approval commitments. The approval of palbociclib (Ibrance) for male breast cancer, based on EHR and claims data, was a landmark case showing how RWE can efficiently extend a therapy’s reach to new populations without a dedicated clinical trial. Guidance is available from sources like the FDA’s framework for RWE.
By accelerating decisions and improving outcomes, real-time evidence generation is now essential to modern pharmaceutical development. For more on this, explore our article on Real-World Data Clinical Trials.
Avoid Costly RWE Failures: Fix Data, Bias, and Privacy to Pass FDA/EMA
While the promise of real-time evidence generation is immense, it comes with significant challenges. The very nature of RWD—collected for clinical care, not research—creates obstacles that must be addressed to ensure credible evidence.
Key challenges include:
- Data Quality and Completeness: RWD often contains missing values, coding inconsistencies (e.g., financial upcoding in claims), and inaccuracies that can introduce bias. Assessing whether data is “fit-for-purpose” for a specific research question is a critical first step.
- Data Gaps and Interoperability: Patient information is frequently siloed across different systems using proprietary formats, making it difficult to create a complete longitudinal record without extensive harmonization.
- Confounding Bias: Unlike in RCTs, observational studies are vulnerable to hidden variables. A key example is “confounding by indication,” where sicker patients are more likely to receive a new drug, making it appear less effective. Advanced statistical methods like propensity score matching are required to create comparable groups and mitigate this bias.
- Patient Privacy and Confidentiality: Protecting sensitive patient data is non-negotiable. This requires strict de-identification, adherence to regulations like HIPAA and GDPR, and the use of privacy-preserving technologies like federated data networks that bring the analysis to the data. At Lifebit, our federated platform is built on these principles.
- Skills Gap: There is a shortage of professionals with expertise in both clinical science and advanced data analytics. Only 20% of leading pharmaceutical companies have an integrated evidence generation plan, highlighting this strategic gap.
For a deeper look, explore our guide on the Challenges of Using Real-World Data in Research.
How Regulatory Bodies Are Adapting
Fortunately, regulatory bodies worldwide are embracing RWE and building frameworks to support its use.
- The US Food and Drug Administration (FDA), guided by the 21st Century Cures Act, established a comprehensive Real-World Evidence Program. Its framework for RWE and subsequent guidance documents detail how to assess RWD fitness-for-purpose and when RWE can support regulatory decisions. Learn more about US Regulatory Guidance on Using Real-World Data.
- The European Medicines Agency (EMA) is launching the DARWIN EU (Data Analysis and Real World Interrogation Network). This federated network of healthcare data sources will allow the EMA and national authorities to commission on-demand studies, enabling faster, more robust real-time evidence generation to support decision-making across the EU. Read An overview of the EMA’s vision for RWE.
- Other bodies like the UK’s NICE and Health Canada are also developing frameworks to integrate RWE into health technology assessments and regulatory decisions.
These advancements signal a fundamental shift, opening the door for real-time evidence generation to become standard practice in healthcare innovation.
Act Now: AI and Federated Data Will Decide Who Wins in Real‑Time Evidence
The trajectory of real-time evidence generation points towards a future where healthcare is more precise, proactive, and patient-centric. This change is happening now, driven by several key trends.

- Predictive Analytics and AI-Driven Discovery: AI algorithms are analyzing massive RWD sets to identify drug targets, predict molecular efficacy, and simulate trials, cutting years and billions from development timelines.
- Personalized Medicine at Scale: Clinicians can compare an individual’s data against global RWD in real-time to find what worked best for “patients like me,” enabling truly customized treatment decisions.
- Decentralized and Hybrid Trials: By combining the rigor of RCTs with the generalizability of RWE, these new trial models allow patients to participate from home using wearables and apps, accelerating research.
- Continuous Learning Health Systems: The ultimate goal is a system that learns from every patient interaction. This creates a virtuous cycle: clinical data is collected, analyzed in near-real-time to generate new evidence, and that evidence is fed back into clinical decision support tools. The outcomes of these improved decisions are then captured as new data, creating a perpetual feedback loop of improvement.
- Global Data Collaboration: Federated networks, like the Lifebit platform, enable secure analysis across institutions and countries without moving sensitive data. This is transformative for rare disease research and generating robust evidence from diverse populations.
- Digital Health Technologies: Wearables and mobile apps are generating unprecedented streams of patient data, capturing real-time insights into daily life, adherence, and quality of life that were previously invisible.
As McKinsey’s vision for medical affairs in 2030 highlights, the future requires more adaptable evidence-generation strategies. Organizations that accept real-time evidence generation now will lead the way in delivering better patient outcomes and accelerating innovation.
Real‑Time Evidence, Fast: Clear Answers to Your Top Questions
Here are answers to the most common questions about real-time evidence generation.
What is the main difference between Real-World Data (RWD) and Real-World Evidence (RWE)?
Real-World Data (RWD) is the raw, unprocessed health data collected from sources like EHRs, claims, and wearables. Real-World Evidence (RWE) is the clinical insight generated after that data is rigorously analyzed. In short, data becomes evidence through analysis.
Can RWE replace Randomized Controlled Trials (RCTs)?
No, RWE is a complement to, not a replacement for, RCTs. RCTs establish efficacy in ideal, controlled conditions (“can it work?”). RWE demonstrates effectiveness in diverse, real-world populations (“does it work in practice?”). RWE fills critical gaps by capturing long-term outcomes and results from patients often excluded from trials. The two approaches work best together.
How is patient privacy protected when using RWD?
Protecting patient privacy is a fundamental requirement. It is achieved through several layers:
- De-identification and Anonymization: All personally identifiable information is removed or encrypted before data is used for research.
- Regulatory Compliance: Strict adherence to governance frameworks like HIPAA in the US and GDPR in Europe is mandatory.
- Privacy-Preserving Technology: Federated learning allows analysis to be performed where data resides, without moving or centralizing sensitive information. Algorithms travel to the data, not the other way around. Trusted Research Environments (TREs) also provide secure, controlled access for authorized researchers.
At Lifebit, our federated platform is built on these privacy-first principles, enabling large-scale real-time evidence generation without compromising patient confidentiality.
Move Now or Fall Behind: Build Real‑Time Evidence Regulators Trust
Real-time evidence generation represents a fundamental shift in how we turn patient data into life-saving insights. It moves beyond the limitations of traditional clinical trials —their high costs, long timelines, and narrow patient populations —to provide a faster, more inclusive, and more accurate understanding of how treatments work in the real world.
The benefits are clear: accelerated innovation, improved patient outcomes, faster regulatory approvals, and more equitable healthcare. This is all made possible by a technological backbone of advanced analytics, AI, and federated learning, which can analyze vast, distributed datasets while ensuring patient privacy and regulatory compliance.
This is where Lifebit’s work is critical. Our federated AI platform was built to solve this exact challenge. We provide secure data access to global networks without moving data. Our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) deliver the advanced AI analytics needed to generate regulatory-grade evidence while upholding the highest standards of data governance.
Biopharma companies, regulatory agencies, and public health organizations trust our technology to power their most critical research. The revolution in evidence generation is here. The question is whether your organization will lead this change or be left behind. Every day of delay means slower innovation and patients waiting for life-changing treatments.
Ready to lead the change? Explore a next-generation federated biomedical data platform and find how Lifebit is making the future of healthcare a reality today.

