AI for biomarker discovery: 2 Exabytes Breakthrough
Open uping Insights: The Role of AI for Biomarker Findy
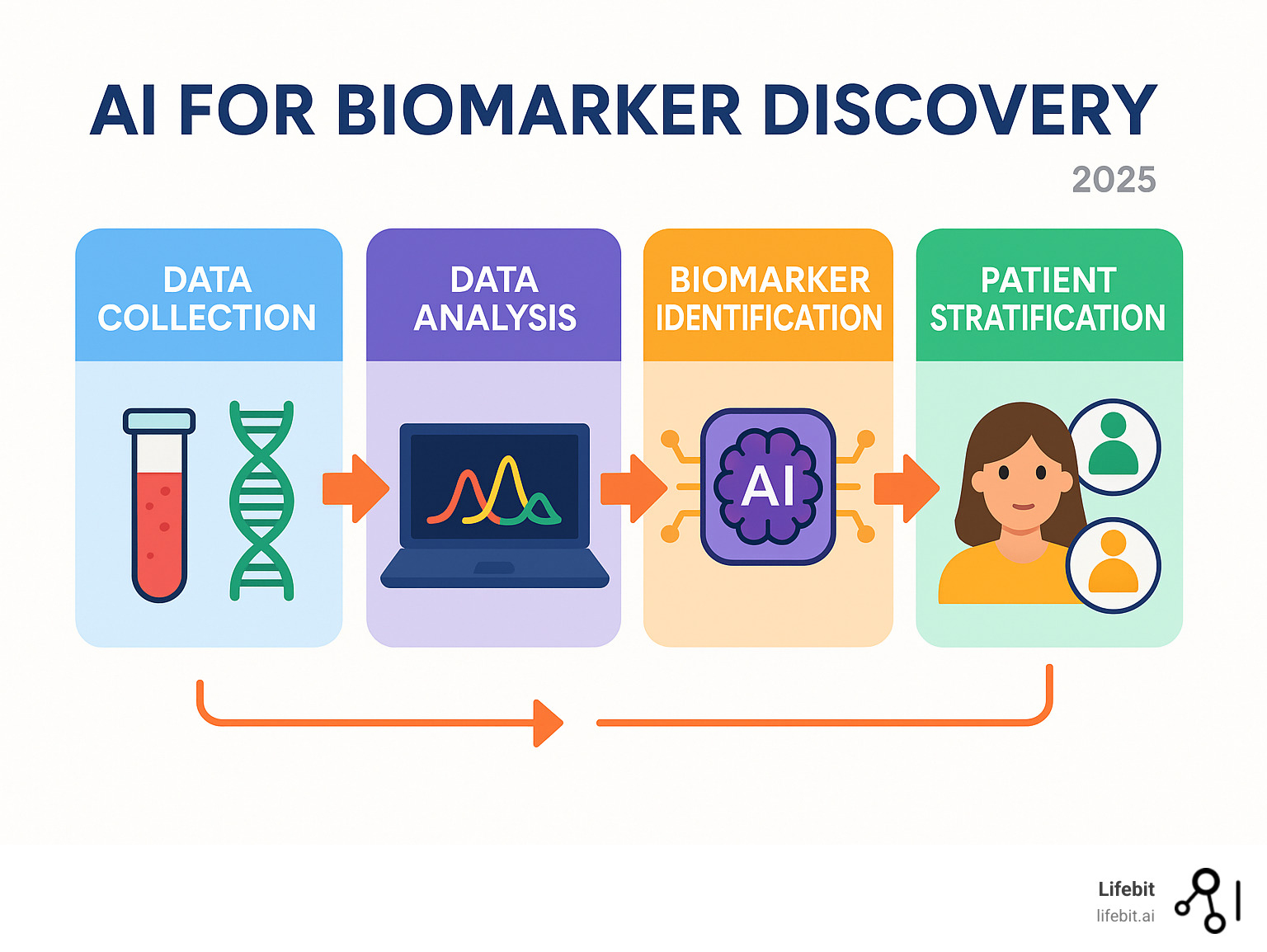
AI for biomarker findy is reshaping how we understand and fight diseases like cancer. It helps researchers find crucial biological signals that indicate a patient’s health, disease progression, or treatment efficacy.
Here’s how AI for biomarker findy helps:
- Analyzing massive datasets: AI processes vast, complex information, from genetic codes to medical images.
- Finding hidden patterns: It uncovers marker combinations that human analysis often misses.
- Predicting outcomes: AI forecasts disease progression, identifies effective treatments, and pinpoints responsive patients.
- Enabling personalized medicine: This leads to treatments custom to each patient.
Finding reliable biomarkers is a major challenge. Diseases like cancer are incredibly complex. For instance, while immune checkpoint inhibitors (ICIs) have transformed cancer treatment, selecting the right patients remains difficult. Traditional single-marker methods often fall short, with experts calling the “one-molecule (or process) marker” an utopia.
This is where AI excels. Handling the vast, diverse data—an estimated 2 exabytes of cancer data were generated in the US alone between 2014 and 2018—is impossible without advanced tools. AI, particularly machine learning, sifts through this data to find ‘meta-biomarkers’ that offer clearer insights. The field’s rapid growth is striking, with 80% of relevant studies published in 2021-2022.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years of experience in computational biology, AI, and health-tech, my work focuses on using high-performance computing and secure data platforms to advance AI for biomarker findy and bring personalized medicine closer to patients.
AI for biomarker findy terms at a glance:
The Challenge: Why We Need Better Predictive Biomarkers
Imagine telling a cancer patient their expensive, life-altering immunotherapy didn’t work. This happens far too often. While immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment, we’re still struggling to predict which patients will actually benefit from these life-saving therapies.
The challenge isn’t efficacy, but complexity. Cancer is an incredibly intricate and heterogeneous disease, and our current prediction methods are often too simplistic. It’s like trying to predict a movie’s box office success based only on its genre, ignoring the cast, director, script, and marketing.
For decades, the search for a perfect single biomarker—the ‘one-molecule marker’ utopia—has focused on predictors like PD-L1 expression, tumor mutational burden (TMB), and microsatellite instability (MSI). While valuable in some contexts, these markers, when used in isolation, often fall short. Cancer requires a more nuanced, holistic approach.
Current Limitations in Immunotherapy Response Prediction
The current state of immunotherapy prediction faces several significant problems that affect patients, clinicians, and healthcare systems alike:
Patient stratification is inconsistent across different cancer types and even within the same diagnosis. This clinical ambiguity leads to a trial-and-error approach, where some patients receive expensive and potentially toxic treatments with little chance of benefit, delaying more suitable alternatives and incurring huge financial and emotional costs.
Existing biomarkers show frustrating variability in their predictive power.
- PD-L1 expression, a widely used marker, is notoriously unreliable. Its expression can change over time, vary significantly between a primary tumor and its metastases, and even differ between parts of the same tumor. Furthermore, testing is not standardized, with different antibody clones and scoring cut-offs leading to conflicting results.
- Tumor Mutational Burden (TMB), the number of mutations in a tumor’s DNA, has also shown promise, but its utility is debated. While a high TMB can correlate with better response, many patients with low TMB still benefit from ICIs, and vice-versa. The lack of a standardized method for calculating TMB further complicates its clinical use.
- Microsatellite Instability (MSI) is a strong predictor of response, but it is only relevant for a small subset of cancer patients, limiting its broad applicability.
Tumor heterogeneity adds another layer of complexity. Different regions within a single tumor can have distinct genetic and molecular profiles, meaning a biopsy from one area may not represent the entire tumor. This makes a single-biomarker approach akin to reading only one page of a book and trying to guess the entire plot.
The dynamic tumor microenvironment (TME)—an intricate ecosystem of cancer cells, immune cells, blood vessels, and signaling molecules—constantly evolves. The TME can be classified into different phenotypes, such as “inflamed” (hot tumors with abundant T-cells), “immune-excluded” (T-cells at the periphery), or “immune-desert” (cold tumors lacking T-cells). A single marker cannot capture the complexity of this ecosystem, which is a critical determinant of immunotherapy success.
Perhaps most concerning is the high cost of ineffective treatments. Immunotherapies can cost hundreds of thousands of dollars per patient annually. When these treatments fail, we waste not only finite healthcare resources but also a patient’s precious time, which could have been spent on a more effective therapy.
How AI for Biomarker Findy Addresses These Challenges
This is where AI for biomarker findy becomes a game-changer. Think of AI as a master detective, capable of examining thousands of clues simultaneously—a scale of investigation that would completely overwhelm human researchers.

High-dimensional data analysis is AI’s superpower. While traditional statistical methods might struggle with dozens of variables, AI algorithms can process millions of data points from thousands of patients simultaneously. It’s the difference between looking at the sky with a magnifying glass and using the James Webb Space Telescope.
Pattern recognition capabilities allow AI to identify subtle, non-linear relationships hidden within complex data. It can spot intricate connections between genes, proteins, cell locations, and clinical outcomes that could take researchers decades to uncover through traditional hypothesis-driven research, if they are found at all.
Integrating disparate data sources is perhaps AI’s most valuable contribution. It can weave together genomics, imaging, pathology, and clinical data to create a holistic, multi-dimensional patient profile that is far more predictive than any single data type.
Accelerating research is a critical outcome. The explosion of interest in this field is a testament to its potential; an impressive 80% of studies in AI for biomarker findy were published in the short span of 2021-2022, reflecting the urgent need and promising results.
The shift from single biomarkers to AI-driven ‘meta-biomarkers’ represents a fundamental paradigm shift in personalized medicine. It’s the difference between hearing a single violin and experiencing the rich, complex harmony of an entire orchestra.
Open uping Insights: Data Modalities and AI Techniques
The world of AI for biomarker findy is buzzing with activity. A systematic review of 90 studies focused on using AI to predict immune checkpoint inhibitor (ICI) effectiveness highlights this rapid growth. The review found that non-small-cell lung cancer (NSCLC) was the most studied malignancy (36% of studies), followed by melanoma (16%), indicating where the clinical need is most urgent and where AI is beginning to make its biggest impact.
AI’s power stems from its ability to learn from vast and diverse data. To fuel these breakthroughs, AI for biomarker findy relies on multiple data types, or modalities, each offering a unique window into a patient’s disease.
The Fuel for AI: Key Data Modalities
For AI for biomarker findy to flourish, it needs rich, diverse, and high-quality data. Think of AI as a master chef and data as the essential ingredients; the more varied and high-quality the ingredients, the more sophisticated and predictive the final creation can be.
First, Multi-omics. This broad category includes:
- Genomics: Data from DNA sequencing (e.g., Whole Exome or Whole Genome Sequencing) that reveals the genetic blueprint of a tumor, including mutations that could drive cancer growth.
- Transcriptomics: Information about actively transcribed genes (via RNA sequencing), which shows which parts of the genetic code are currently active and influencing cell behavior.
- Proteomics: The large-scale study of proteins, which are the functional workhorses of cells. This provides a more direct look at cellular processes than genomics alone.
- Metabolomics: The analysis of small molecules, or metabolites, which provides a real-time snapshot of a cell’s physiological state.
Next is Radiomics, the science of extracting vast quantities of quantitative features from standard medical images like CT, MRI, and PET scans. AI algorithms can identify and analyze hundreds of features—related to tumor shape, size, intensity, and texture—that are invisible to the human eye, offering powerful clues about tumor characteristics, heterogeneity, and the surrounding microenvironment.
Then there’s Pathomics, also known as Digital Pathology. By digitizing whole-slide images of tissue samples at high resolution, AI can perform exhaustive analysis of cellular morphology, tissue architecture, and the spatial relationships between different cell types. This allows for the findy of novel “spatial biomarkers,” such as the precise location and density of tumor-infiltrating immune cells, which is a critical factor in immunotherapy response.
Crucially, Real-world data (RWD) from sources like electronic health records (EHRs), insurance claims, and disease registries provides a longitudinal view of patient health, treatment history, and outcomes outside the controlled environment of a clinical trial. This includes unstructured data from clinical notes, which can be mined using Natural Language Processing (NLP) to extract rich contextual information.
But the real magic happens with Multimodal data, where AI for biomarker findy integrates these different data types. Blending genomic data with radiomic features, for instance, can create a far more complete and predictive model than either source could provide alone. Many foundational datasets are accessible to researchers through public repositories like The Cancer Genome Atlas (TCGA).
The Engine: Common AI and Machine Learning Methods
With rich datasets as fuel, we need the right AI “engine” to power the findy process. The aforementioned review found that 72% of studies used Standard Machine Learning (ML), 22% used Deep Learning (DL), and 6% used a hybrid approach.
Here’s a comparison of common AI methods often used in AI for biomarker findy:
| Method Category | Examples | Strengths | Weaknesses |
|---|---|---|---|
| Standard ML | Random Forest (RF), Support Vector Machines (SVM), Logistic Regression, K-Nearest Neighbors (KNN), Gradient Boosting Machines (GBM) | Often simpler, more interpretable, require less data for training, computationally less intensive, effective for structured data. | May struggle with very high-dimensional or unstructured data (like raw images), can miss complex non-linear relationships, performance might plateau with massive datasets. |
| Deep Learning | Convolutional Neural Networks (CNNs), Vision Transformers (ViTs), Recurrent Neural Networks (RNNs), Graph Neural Networks (GNNs) | Excellent at learning complex patterns from raw, unstructured data (images, sequences), can handle massive datasets, often achieve state-of-the-art performance. | Require very large datasets for optimal performance, often “black box” models (less interpretable), computationally intensive (often needing GPUs), prone to overfitting if not carefully managed. |
For example, Convolutional Neural Networks (CNNs) are perfectly suited for analyzing image data, making them the go-to choice for radiomics and pathomics. More recently, Vision Transformers (ViTs) have shown impressive results by processing images in a novel way, treating patches of an image like words in a sentence.
Graph Neural Networks (GNNs) are uniquely powerful for modeling complex relationships, such as gene-gene interaction networks or the spatial arrangement of cells in the tumor microenvironment. Finally, specialized Survival analysis models are essential for the ultimate goal: predicting patient outcomes over time, such as overall survival or disease-free progression, which is the key validation for any predictive biomarker.
The Breakthrough: Finding ‘Meta-Biomarkers’ with Multimodal AI
The true frontier in AI for biomarker findy is the creation of ‘meta-biomarkers.’ These are not single, isolated data points but sophisticated, composite signatures that emerge from the intelligent fusion of multiple data types. Just as a modern weather forecast is far more accurate because it integrates data from satellites, ground sensors, and atmospheric models, a meta-biomarker provides a more robust and reliable prediction.
This is the power of multimodal data integration. For example, an AI model might determine that patients with a specific mutation (genomics), high lymphocyte infiltration in a specific tumor region (pathomics), and a certain tumor texture on a CT scan (radiomics) have a 90% response rate to a particular ICI. No single marker provides this clarity, but their AI-driven integration creates a highly accurate predictive tool.
So, what are meta-biomarkers? They are novel, high-performance biomarkers derived from the synergistic analysis of multi-omic and multimodal data. They represent a deeper, more systems-level understanding of biology, capturing the intricate interplay between different biological layers.
This integration leads to significantly improved predictive power. By building more robust and accurate models, AI enables the precise patient stratification needed to match the right patient to the right treatment at the right time. This multimodal approach is not just an advantage but a necessity for advancing complex treatments like immunotherapies, which demand a comprehensive biological understanding that single-biomarker methods simply cannot provide. You can read more on multimodal biomedical AI.
From Research to Reality: The Path to Clinical Integration
Translating a brilliant research finding into a life-changing clinical tool is a monumental leap. While the promise of AI for biomarker findy is immense, the journey from a published paper to a trusted instrument in a doctor’s hands is fraught with real-world challenges.
This journey requires far more than technical breakthroughs; it demands rigorous validation, the establishment of clinical trust, and the creation of clear pathways for regulatory approval and adoption. The ultimate goal is to enable smarter clinical trials, find novel therapeutics, and deliver truly personalized care to every patient.
Overcoming Problems in Clinical Translation
Despite impressive academic strides, the road to routine clinical adoption for AI-finded biomarkers is a steep one. Like a newly designed bridge, these powerful tools must be stress-tested from every angle to prove they are safe, reliable, and effective. Here are the main problems:

First is the lack of high-level evidence from prospective studies. Many promising AI studies are retrospective, meaning they analyze existing, historical data. While essential for generating hypotheses, this doesn’t prove a model’s value in a real-world clinical workflow. True clinical adoption requires prospective trials where the AI biomarker is used to make decisions for new patients in real time.
Second is the challenge of model generalizability and bias. Deep learning models require vast, diverse datasets to learn effectively. A model trained on data from a single hospital or a specific patient demographic may fail spectacularly when applied to a different population. This makes small sample sizes a major barrier and underscores the critical need for globally diverse data to ensure AI tools are equitable and work for everyone. Standardized reporting guidelines like CONSORT-AI are essential for transparency.
Third, data quality and standardization are persistent obstacles. AI models are sensitive to variations in how data is collected. Different MRI scanner settings, tissue staining protocols, or DNA sequencing platforms can introduce technical artifacts that confuse a model. Overcoming this requires significant effort in data harmonization and the adoption of standardized protocols across institutions.
Finally, navigating regulatory pathways is a complex process. An AI biomarker is often considered “Software as a Medical Device” (SaMD) by bodies like the U.S. FDA and the European Medicines Agency. Gaining approval requires extensive documentation and validation to prove both analytical validity (the model is accurate and reliable) and clinical validity (the model’s output is meaningful for the patient’s condition).
The Importance of Explainable AI (XAI) for Biomarker Findy
Imagine a doctor recommending a major treatment based on an AI’s suggestion but being unable to explain why the AI made that choice. This is the “black box” problem inherent to many powerful AI models: they can provide stunningly accurate answers, but their internal reasoning is often opaque.
This is where Explainable AI (XAI) becomes absolutely vital for AI for biomarker findy. XAI is a set of techniques designed to make AI models transparent and their decisions understandable to humans. This is essential for building clinical trust; clinicians must be able to understand and verify an AI’s logic before they can confidently and ethically incorporate it into patient care.
While there can be a trade-off between a model’s performance and its interpretability, modern XAI techniques aim to bridge this gap. For example, saliency maps can highlight the specific pixels in a medical image that an AI model found most important. More advanced methods like LIME (Local Interpretable Model-agnostic Explanations) can explain a prediction for a single patient, while SHAP (SHapley Additive exPlanations) can reveal which biomarkers are driving predictions across the entire patient population. This not only builds trust but can also lead to new scientific findies by pointing researchers toward previously unknown biological signals. You can learn about Explainable AI concepts.
Future Directions for AI for Biomarker Findy
The future for AI for biomarker findy is incredibly bright. As we refine our models and overcome translational challenges, several key areas are ready for transformative growth:
First, AI will revolutionize patient selection for clinical trials. By precisely identifying the patient subgroups most likely to benefit from an investigational therapy, AI can lead to smaller, faster, and more successful trials, dramatically accelerating the pace of drug development.
AI will also be instrumental in novel therapeutic target identification. By analyzing complex biological networks in multi-omic data, AI can pinpoint new genes, proteins, or pathways that are critical for disease progression, opening up entirely new avenues for drug findy and repurposing.
We are also moving towards dynamic monitoring of treatment response. This will likely be powered by serial analysis of liquid biopsies, which can detect circulating tumor DNA (ctDNA) in a patient’s blood. AI models can track changes in ctDNA over time to provide a real-time assessment of treatment efficacy, allowing doctors to detect resistance early and adjust treatments on the fly.
Seamless integration with electronic health records (EHR) will be another game-changer. When AI tools are embedded directly into clinical workflows, they can pull and analyze data in real time, delivering actionable insights to clinicians at the point of care and making AI for biomarker findy a routine part of modern healthcare.
Finally, federated learning is emerging as a powerful solution to data privacy and access challenges. This approach trains AI models on decentralized data without the data ever leaving its secure, local environment. The model is sent to the data, trained locally behind an institution’s firewall, and only the anonymous model updates are sent back to be aggregated. At Lifebit, our federated AI platform is built for this purpose, enabling secure, large-scale research across global biomedical data. Our platform’s components, like the Trusted Research Environment (TRE) and R.E.A.L. (Real-time Evidence & Analytics Layer), deliver real-time insights and secure collaboration, powering the next generation of biomarker findy while keeping patient data safe. You can find more info about our federated AI platform.
Frequently Asked Questions about AI for Biomarker Findy
You’ve explored how AI for biomarker findy is revolutionizing medicine. Let’s answer some common questions about this exciting field.
What is the main advantage of using AI over traditional methods for biomarker findy?
Traditional methods are like finding a few pieces in a 100-piece puzzle—doable, but slow. Modern biology is like a puzzle with billions of mixed-up, multi-dimensional pieces.
AI excels at sifting through these massive, complex datasets—genomics, imaging, and clinical records—simultaneously. It spots hidden patterns that older methods miss, helping us find powerful ‘meta-biomarkers.’ These combinations of markers provide a clearer picture, leading to better predictions and diagnoses.
Which cancer types are most studied for AI-driven biomarker findy?
Based on recent research for AI for biomarker findy, two cancer types stand out. Non-small-cell lung cancer (NSCLC) leads, appearing in 36% of studies, followed by melanoma at 16%.
These cancers are a focus because while immunotherapies have been transformative, not all patients respond. There is an urgent need to use AI to better predict who will benefit from these treatments.
Are AI-finded biomarkers ready for clinical use today?
The short answer is: not quite yet for widespread use. While AI for biomarker findy shows incredible promise in retrospective studies (analyzing existing data), these findings are just the first step.
For an AI-finded biomarker to become a standard clinical tool, it needs rigorous testing in prospective clinical trials. These studies test the AI in real-time on new patients to confirm its safety and efficacy. This validation ensures the insights are reliable and can improve patient care. It’s a journey from lab to clinic, and we’re making great progress!
Conclusion
The future of diagnostics, led by AI for biomarker findy, is incredibly exciting. We are moving beyond the search for a single “magic bullet” biomarker to finding powerful “meta-biomarkers”—complex signatures built from combining vast, diverse data.
This future is multimodal, leveraging rich information from genomics, radiomics, pathomics, and real-world data from health records.
This future is also explainable. We focus on Explainable AI (XAI) to build clinical trust by making AI’s reasoning transparent. Finally, this future is rigorously validated. We are committed to putting AI-driven insights through clinical trials, turning research into actionable tools for patients.
At Lifebit, we are proud to be part of this revolution. Our next-generation federated AI platform is designed to enable the secure, real-time, large-scale analysis of vital multimodal data needed to power the next wave of AI for biomarker findy. By facilitating secure collaboration and offering advanced AI/ML capabilities, we accelerate the findy of crucial meta-biomarkers, helping to optimize immunotherapy and personalize cancer treatment.
We believe combining biomarkers with advanced AI and ML algorithms will open up unprecedented breakthroughs in cancer diagnostics, prognostics, and treatment decisions. This is about bringing more precise, effective, and personalized treatments to patients worldwide.
Ready to see how we can help you improve your biomarker findy with federated AI? Find out how to improve your biomarker findy with federated AI.

