How Clinical Cancer Research is Changing the Game

Clinical Cancer Research: Breakthroughs in 2025
Why Clinical Cancer Research Matters More Than Ever
Clinical cancer research is scientific investigation that involves people, their data, or samples from people to develop better ways to prevent, detect, and treat cancer. It includes two main types of studies:
- Clinical trials – Test new treatments, prevention methods, or screening approaches
- Observational studies – Study cancer patterns, risk factors, and outcomes in populations
Clinical cancer research encompasses multiple disciplines including medical oncology, radiation therapy, surgical oncology, pharmacology, immunology, and molecular genetics. All studies aim to improve patient outcomes and advance our understanding of cancer.
The field has evolved dramatically since the 1930s when chemotherapy first emerged. Today, groundbreaking findies like CAR-T cell therapy for blood cancers and the HPV vaccine for cervical cancer prevention demonstrate how clinical research directly saves lives.
Current statistics paint both the challenge and the opportunity ahead:
- Cancer affects approximately 19.3 million people globally each year
- The disease accounts for roughly one in six deaths worldwide
- By 2025, experts project the global cancer burden will reach nearly 20 million new cases annually
Yet there’s unprecedented hope. Modern clinical cancer research has produced remarkable advances in immunotherapy, targeted therapies, and precision medicine approaches that tailor treatments to individual patients’ genetic profiles.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, with over 15 years of experience in computational biology and genomics that directly supports clinical cancer research through secure, federated data analysis platforms. My work focuses on accelerating cancer research breakthroughs by enabling researchers to collaborate across institutions while maintaining data privacy and regulatory compliance.
Clinical cancer research terms simplified:
Understanding the Landscape of Clinical Cancer Research
Think of clinical cancer research as a massive puzzle where every piece matters. It’s the field where brilliant minds work together to understand cancer better and find ways to beat it. At its heart, this research is all about taking what we learn in labs and turning it into real help for patients.
The scope of this work is truly impressive. We’re talking about everything from studying cancer cells under microscopes to testing new treatments in hospitals. It’s like having detectives, engineers, and doctors all working on the same case – each bringing their special skills to solve the mystery of cancer.
Medical oncologists are the treatment specialists who work directly with patients. Radiation therapists use high-energy beams to target tumors. Surgical oncologists are the skilled surgeons who remove cancerous tissue. Each plays a vital role in both patient care and research studies.
But the story doesn’t stop there. Behind the scenes, other experts are making crucial contributions. Pharmacologists study how drugs work in our bodies – they’re the ones figuring out the right doses and combinations. Immunologists explore how our immune system can fight cancer, leading to those exciting immunotherapy breakthroughs we hear about. Molecular geneticists dive deep into our DNA to understand what makes cancer tick and how we can stop it.
It’s like watching a well-orchestrated team where everyone has a special role, but they’re all working toward the same goal: helping people beat cancer.
The Two Main Pillars: Study Types in Clinical Cancer Research
Clinical cancer research relies on two main types of studies, and understanding the difference can help you see how progress happens.
Clinical trials are where we test new ideas. Think of them as careful experiments where patients volunteer to try something new – maybe a promising drug, a different surgical technique, or even a new way to prevent cancer. These studies are highly controlled, with strict rules about who can participate and exactly how the treatment is given.
Participants in clinical trials might take a new medication, undergo a different type of surgery, or receive an innovative vaccine. They also help researchers by providing blood samples, having extra scans, or undergoing genetic tests. It’s all about finding out if new approaches are safe and work better than what we currently have.
Observational studies take a different approach. Instead of testing new treatments, researchers watch and learn from what’s already happening. They might follow thousands of people over many years, tracking their health through surveys and medical records. Or they might study tissue samples to understand why some treatments work better for certain patients.
These studies help us spot patterns. Maybe they’ll find that people who eat certain foods are less likely to get cancer, or that patients with specific genetic markers respond better to particular treatments.
Both types of studies depend on people willing to participate. Whether someone has cancer now, survived it in the past, or is perfectly healthy, their contribution helps move research forward.
| Feature | Clinical Trials | Observational Studies |
|---|---|---|
| Goal | Test new interventions (drugs, procedures, prevention) | Observe patterns, risk factors, outcomes |
| Patient Action | Receive specific intervention, provide samples/data | Provide data (surveys, records), samples (blood, tissue) |
| Example Activity | Take a new drug, have a new surgery, get a vaccine | Fill out a health survey, donate tumor tissue |
| Primary Focus | Safety and efficacy of new treatments | Associations between factors and health outcomes |
From the Lab to the Clinic: The Role of Translational Research
Here’s where things get really exciting. Translational research is the bridge that connects brilliant lab findies with real treatments for patients. Scientists call this the “bench-to-bedside” concept – taking something that works in a petri dish and making it work in people.
The journey is fascinating but complex. Let’s say researchers find that cancer cells have a specific weakness – maybe they depend on a particular protein to survive. Scientists then design a new molecule-targeted agent that can block this protein. First, they test it extensively in lab studies and animal models. Only after it shows promise do they move to human trials.
But translational research works both ways. Sometimes doctors notice something interesting about how patients respond to treatment. Maybe a drug works amazingly well for some people but not others. This observation goes back to the lab, where scientists try to figure out why. They might find a genetic marker that predicts who will benefit most from the treatment.
This back-and-forth between lab and clinic creates a powerful cycle of findy and improvement. Each finding builds on the last, gradually making treatments more effective and personalized.
Publications like Clinical Cancer Research showcase this kind of groundbreaking work. They focus on studies that bridge laboratory science and patient care, featuring everything from early-stage clinical trials to research on biomarkers that help predict treatment responses. You can explore their latest findings at the Homepage of the journal Clinical Cancer Research.
This collaborative approach is what makes modern cancer research so promising. We’re not just throwing treatments at cancer and hoping they work – we’re understanding the disease at a molecular level and designing smart, targeted approaches to beat it.
The Engine of Progress: How Clinical Trials Advance Cancer Care

Think of clinical cancer research as a powerful vehicle moving toward a world without cancer. Clinical trials are the engine that drives this progress forward, one carefully planned study at a time.
Every cancer treatment we rely on today started as an experimental idea in a clinical trial. The chemotherapy drugs that have saved millions of lives? They were once untested compounds. The targeted therapies that can shrink tumors with fewer side effects? They began as promising molecules in a lab. Even immunotherapy treatments that help the body’s own immune system fight cancer had to prove themselves through rigorous clinical testing.
Finding better treatments is just one piece of the puzzle. Clinical trials also help us improve patient outcomes by finding which treatments work best for specific types of cancer, which combinations are most effective, and how to minimize side effects. When a treatment proves successful in trials, it becomes part of the new standard of care that benefits patients everywhere.
The real heroes in this story are patient volunteers. Without people willing to participate in clinical trials, medical progress would grind to a halt. These brave individuals don’t just contribute to science – they often gain access to innovative therapies that aren’t yet available to the general public.
For patients facing aggressive cancers or those who haven’t responded to standard treatments, clinical trials can offer hope when other options have been exhausted. It’s not a guarantee, but it’s a chance to try something new while contributing to knowledge that could help countless others.
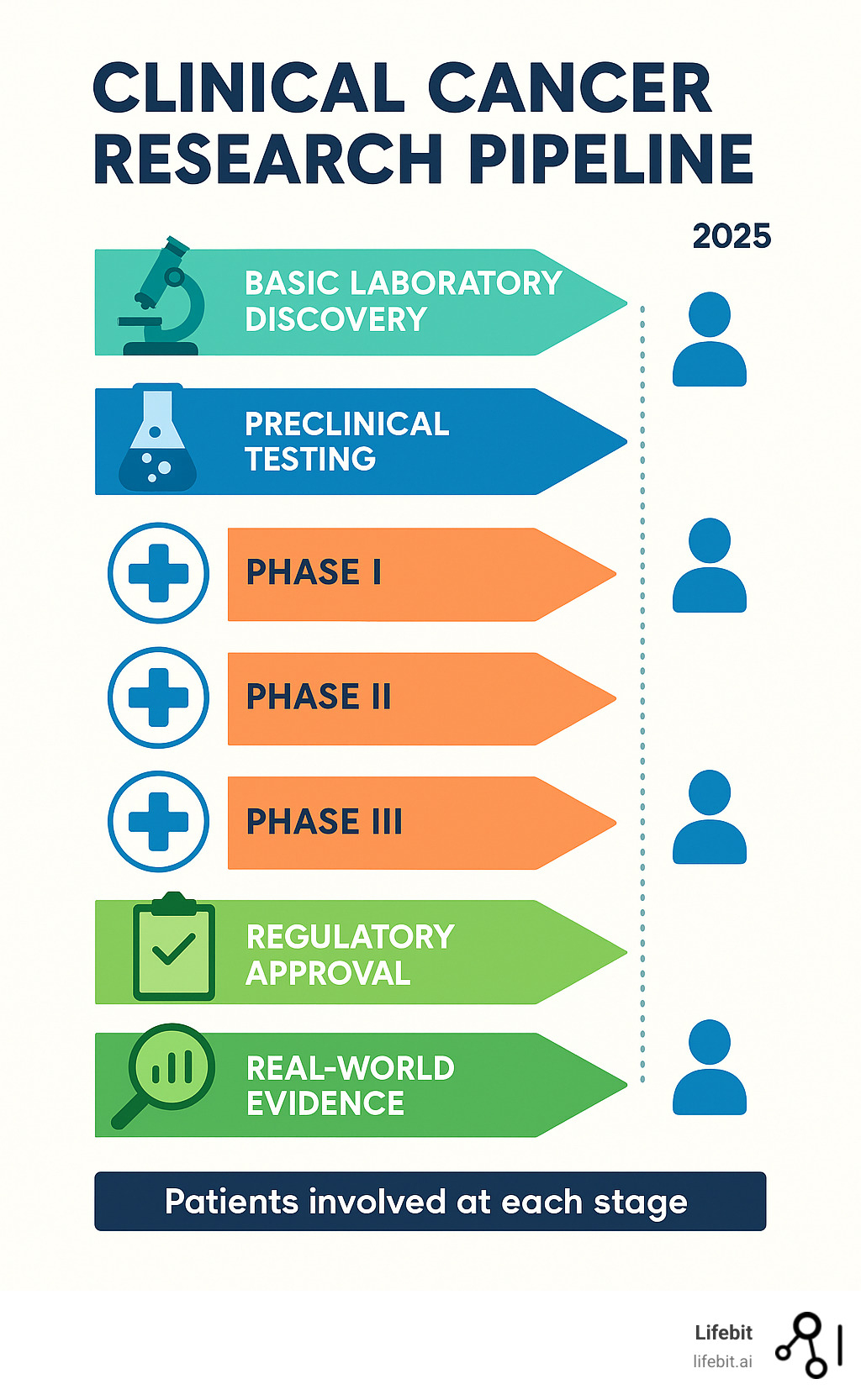
The Phases of a Clinical Trial Explained
Clinical trials follow a careful, step-by-step process that builds knowledge gradually and safely. Think of it like test-driving a new car – you wouldn’t take it on a cross-country road trip without first checking if the brakes work.
Phase I focuses on safety and dosage. This is where researchers first test a new treatment in humans, usually with 20 to 80 people who have advanced cancer and few remaining treatment options. The main questions are simple but crucial: Is this safe? What’s the right dose? How does the body handle this new treatment? Researchers aren’t primarily looking for the treatment to work yet – they just need to know it won’t cause serious harm.
Phase II explores efficacy and side effects. If a treatment passes Phase I safety tests, it moves to a larger group of 100 to 300 people, usually those with a specific type of cancer. Now researchers want to know: Does this actually help patients? Do tumors shrink? Do symptoms improve? They’re also watching carefully for side effects that might not have shown up in the smaller Phase I group.
Phase III provides comparison to standard treatment. This is often the most important phase, involving hundreds or thousands of participants. The new treatment goes head-to-head with the current best treatment available. Participants are randomly assigned to different treatment groups to ensure fair comparison. If the new treatment performs better than the standard care, it’s ready for regulatory approval.
Phase IV continues with post-marketing surveillance. Even after a treatment gets approved and becomes available to patients, the learning doesn’t stop. Phase IV studies track how the treatment performs in the real world, with diverse populations and over longer periods. Researchers might find new uses for the treatment or learn more about long-term effects.
Each phase builds on the last, creating a solid foundation of evidence that helps doctors and patients make informed decisions about cancer care.
Modernizing Trial Design: Beyond the Traditional Phases
While the four-phase model has been the bedrock of clinical cancer research, the rise of precision medicine has spurred the development of more efficient trial designs. These newer models, often called master protocols, accelerate findy by testing multiple drugs or addressing multiple cancer types under a single trial structure.
- Basket Trials: These trials enroll patients with a specific genetic mutation, regardless of where their cancer originated. It’s an efficient way to test a targeted drug, like a BRAF inhibitor, across many cancer types that share the same molecular driver, testing the hypothesis that the marker is more important than the cancer’s location.
- Umbrella Trials: An umbrella trial works in the opposite way. It focuses on a single cancer type, like lung cancer, but tests several different targeted drugs simultaneously. Patients are assigned to a treatment arm based on the molecular alterations found in their tumor, matching the right drug to the right patient.
- Platform Trials: This dynamic design is adaptive, allowing researchers to test multiple interventions against a common control group. New experimental treatments can be added and ineffective ones dropped without starting a new trial from scratch. The I-SPY 2 trial in breast cancer is a landmark example, continuously evaluating new agents for high-risk patients.
The Future is Now: Current Trends and Emerging Areas
We’re living in an incredible time for clinical cancer research. The field is changing at a pace that would have been unimaginable just a decade ago. Innovation in oncology is happening everywhere – from the lab bench to the patient’s bedside – and it’s genuinely exciting to witness.
The most remarkable shift we’re seeing is the move away from treating all cancers the same way. Instead of the old “one-size-fits-all” approach, we’re embracing precision medicine – where treatments are custom to each patient’s unique cancer profile. It’s like moving from using a sledgehammer to using a precision scalpel.
What’s driving this revolution? Big data and artificial intelligence are game-changers. Today’s research generates enormous amounts of information – genetic data, protein patterns, treatment responses, and more. AI helps us find patterns in this data that human researchers could never spot on their own. This isn’t science fiction anymore; it’s happening right now in cancer centers around the world.
The Power of Personalized Medicine and Biomarkers
Here’s where things get really fascinating. Biomarkers are like molecular fingerprints that tell us crucial information about a patient’s cancer. Think of them as biological clues that help doctors make smarter treatment decisions.
These biomarkers can be anything from a specific gene mutation in tumor tissue to protein levels in a blood sample. What makes them so powerful is their ability to predict treatment response. Instead of trying different treatments and hoping for the best, doctors can now look at these molecular signals to choose therapies that are most likely to work for each individual patient.
Targeted therapy is where personalized medicine really shines. These drugs are designed like smart missiles – they seek out and attack specific vulnerabilities in cancer cells while largely leaving healthy cells alone. Some targeted therapies block overactive proteins that fuel cancer growth, while others deliver chemotherapy directly to cancer cells like a Trojan horse.
The world of immunotherapy has been revolutionized by biomarkers too. Take PD-L1, for example. This protein can tell doctors whether a patient’s cancer is likely to respond to immune checkpoint inhibitors – drugs that essentially remove the brakes from the immune system so it can recognize and destroy cancer cells more effectively.
One of the most exciting developments is the use of molecular alterations to guide treatment. Through advanced genetic testing, doctors can now identify the specific mutations driving a patient’s cancer and match them with targeted therapies. It’s like having a detailed blueprint of the enemy’s weaknesses.
Key Challenges and Future Directions in Clinical Cancer Research
Despite all this progress, we’re not without our challenges. Drug resistance remains one of our biggest opponents. Cancer cells are incredibly adaptable – they can develop workarounds to even our best treatments. This means we’re constantly in an arms race, developing new therapies and combination approaches to stay one step ahead. Researchers are tackling this challenge head-on, as highlighted in studies of novel therapeutic agents in clinical trials, where scientists are exploring innovative compounds and strategies to overcome resistance mechanisms.
Data access and security present another complex puzzle. Clinical cancer research generates incredibly sensitive patient information that needs to be protected, yet researchers across different institutions need to collaborate and share insights. This is where federated learning becomes crucial – it allows researchers to train AI models on data from multiple locations without the sensitive information ever leaving its secure home.
We also face the critical challenge of patient recruitment diversity. Clinical trial populations have historically not reflected the real world. This is a scientific problem, as genetic ancestry can influence drug effectiveness. To ensure new therapies work for everyone, research must actively recruit diverse participants by building trust and removing logistical barriers.
Looking ahead, several exciting trends are shaping the future of clinical cancer research. One of the most transformative is the rise of liquid biopsies. These are blood tests that detect circulating tumor DNA (ctDNA). Liquid biopsies offer a minimally invasive way to profile a tumor’s genetics, track treatment response, and identify resistance mutations early, revolutionizing trial monitoring.
Real-world data analysis is expanding beyond traditional clinical trials to include insights from electronic health records and patient registries, giving us a broader picture of how treatments perform in everyday practice.
Collaborative research models are becoming more sophisticated, with academic institutions, biotech companies, and patient advocacy groups working together. The old silos are breaking down, and the pace of findy is accelerating as a result.
The future holds enormous promise, and we’re just getting started.
Frequently Asked Questions about Clinical Cancer Research
When it comes to clinical cancer research, we know you probably have lots of questions swirling around in your mind. That’s completely natural! Cancer research can feel overwhelming, especially when you’re considering participation or just trying to understand how it all works. Let’s tackle some of the most common questions we hear, and hopefully give you the clarity and confidence you’re looking for.
Is it safe to participate in a cancer clinical trial?
This is often the first question on everyone’s mind, and it’s absolutely the right one to ask. Your safety matters above everything else in clinical cancer research, and the medical community takes this responsibility incredibly seriously.
Before you even consider joining a trial, you’ll go through something called informed consent. Think of this as having a really thorough conversation with your research team. They’ll sit down with you and explain everything – what the study is trying to accomplish, what you’ll need to do, what might happen (both good and not-so-good), and what other options you have. You’re encouraged to ask as many questions as you want, take your time deciding, and remember that you can change your mind and leave the study at any point.
Behind the scenes, there are watchful eyes making sure everything stays safe. Institutional Review Boards (IRBs) are like independent safety committees made up of doctors, scientists, and regular community members. They review every single research plan before it starts to make sure it’s ethical and protects participants. For bigger trials, Data and Safety Monitoring Boards (DSMBs) – groups of independent experts – regularly check the study data to watch for any safety concerns. If they spot something worrying, they can recommend changes or even stop the trial entirely.
Now, let’s be honest – there are always potential risks and benefits with any medical treatment, including those in clinical trials. But all these safety measures work together to make sure the potential benefits outweigh the risks. The key is having open, honest conversations with your doctor and the research team about any concerns you have.
How can I find a cancer clinical trial?
Finding the right clinical cancer research trial might seem like searching for a needle in a haystack, but there are actually some fantastic resources to help guide your way.
Talking to your doctor is usually your best starting point. Your oncologist knows your medical history inside and out, and they’re perfectly positioned to help you identify trials that might be a good match for your specific type of cancer and situation. They often have connections with research centers and can point you in the right direction.
The National Cancer Institute (NCI) resources are absolutely invaluable. Their website at clinicaltrials.gov is like a massive, searchable library of cancer clinical trials. You can filter by cancer type, location, and other factors that matter to you. It’s completely free and updated regularly.
Patient advocacy groups are another wonderful resource. Many organizations focused on specific types of cancer maintain their own lists of relevant trials and can provide guidance on navigating the whole process. They understand the patient perspective and can offer both practical help and emotional support.
There are also various online trial finders beyond the government site. A targeted search can often turn up additional options and resources.
When you’re searching, it helps to have your cancer diagnosis details handy, information about previous treatments you’ve had, and any specific genetic mutations or biomarkers that have been identified in your tumor. This information will help narrow down the options to trials where you might actually be eligible.
How is the impact of cancer research measured?
You might wonder how we actually know if clinical cancer research is making a real difference. It’s a great question, and the answer involves several layers of evaluation that help us understand progress and recognize truly significant contributions.
Everything starts with the peer-review process. Before any research study gets published, other experts in the field put it through rigorous scrutiny. They examine the methods, question the conclusions, and make sure the work meets high scientific standards. It’s like having your work checked by the toughest teachers before it gets shared with the world.
Once research passes peer review, it gets published in scientific journals. These journals have their own reputation scores that help us understand their influence. The Impact Factor measures how often articles from a particular journal get cited by other researchers – basically, how much attention and credibility the work receives. The SCImago Journal Rank (SJR) is even more sophisticated, considering not just how many citations a journal gets, but where those citations come from. A mention in a highly respected journal carries more weight.
Citation metrics for individual research papers also tell us a lot. When a study gets cited hundreds or thousands of times by other researchers, it usually means it contained important findings that are shaping future work.
Looking at the evolution of research publications gives us insight into how the field is growing and changing. For instance, the journal Clinical Cancer Research started in 1995 with 500 manuscript submissions and grew to nearly 800 by 1998. The fact that it has consistently ranked in the top tier of cancer research journals since 1999 shows the sustained quality and impact of the work it publishes.
But here’s what really matters: the true measure of impact isn’t just numbers and rankings. It’s how research findings actually translate into better treatments, improved patient outcomes, and improved quality of life for people facing cancer. That’s the impact that counts most.
Conclusion
As we reach the end of our exploration into clinical cancer research, it’s remarkable to reflect on just how far we’ve come and how much hope lies ahead. We’ve uncovered the fundamental building blocks of this vital field – from understanding what clinical research actually means to diving deep into the phases of clinical trials that bring new treatments to life.
The change happening in cancer care right now is truly extraordinary. Immunotherapy has revolutionized how we think about treatment, turning the body’s own immune system into a powerful weapon against cancer. Meanwhile, personalized medicine is making the dream of custom treatments a reality, where doctors can look at your tumor’s unique genetic fingerprint and choose therapies specifically designed to target those exact vulnerabilities.
But perhaps what strikes me most is the incredible partnership between patients and researchers that makes all of this possible. Every breakthrough we’ve discussed – from targeted therapies to biomarker-driven treatments – exists because brave individuals chose to participate in clinical trials. Their contributions ripple forward, helping countless others who will face similar battles.
The challenges we face, like drug resistance and ensuring diverse patient representation in trials, aren’t roadblocks – they’re puzzles waiting to be solved. The collaborative spirit driving clinical cancer research today gives me tremendous confidence that we’ll continue finding innovative solutions.
This is where technology becomes truly exciting. At Lifebit, we’re witnessing how federated AI is accelerating breakthroughs in ways we couldn’t have imagined just a few years ago. Our platform enables researchers around the world to collaborate securely, analyzing vast amounts of biomedical data while keeping patient information protected. It’s like having a global research team working together, but with all the privacy safeguards patients deserve.
The beauty of our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) is that they’re not just technical tools – they’re bridges connecting brilliant minds across continents. When researchers can access and analyze data in real-time while maintaining compliance, drug findy accelerates, and patients get access to life-saving treatments faster.
The future of cancer treatment isn’t just bright – it’s blazing with possibility. As we continue pushing the boundaries of what’s possible in clinical cancer research, we’re not just fighting a disease; we’re rewriting the story of what it means to face cancer in the 21st century.
Learn how our platform powers secure, collaborative research

