Why Clinical Research Infrastructure is the Backbone of Medical Progress

Clinical research infrastructure: Key to 2025 Success
Why Clinical Research Infrastructure Powers Medical Breakthroughs
Clinical research infrastructure is the comprehensive network of people, technology, processes, and governance enabling safe, effective medical studies. It includes everything from trained investigators and patient engagement systems to secure data platforms and regulatory frameworks that transform laboratory findings into life-saving treatments.
Key components of modern clinical research infrastructure:
- Human capital: Trained investigators, research coordinators, and engaged patient communities
- Technology platforms: Electronic data capture systems, clinical trial management tools, and secure data environments
- Governance frameworks: Ethics review boards, regulatory oversight, and standardized protocols
- Physical facilities: Clinical research centers, biocontainment labs, and specialized equipment
- Funding mechanisms: Government grants, industry partnerships, and sustainable financing models
The stakes are high. A recent WHO stakeholder consultation revealed significant gaps in clinical research capabilities worldwide. The COVID-19 pandemic exposed this criticality: nations with stronger research networks rapidly tested treatments and vaccines, while others struggled.
Canada’s massive $250 million Clinical Trials Fund investment shows governments recognize this reality. The fund supports 232 researchers across the country through the Accelerating Clinical Trials consortium, proving that coordinated infrastructure investment delivers results. Meanwhile, innovative programs like the University of Utah’s Academic Associate initiative have supported 820 students since 2009, with strong representation from underrepresented groups – showing how infrastructure can drive both research excellence and health equity.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. For over 15 years, I’ve built genomics and biomedical data platforms that strengthen clinical research infrastructure through federated analytics and secure data collaboration. My work creates the digital backbone for researchers to accelerate drug findy while maintaining top-tier data privacy and regulatory compliance.
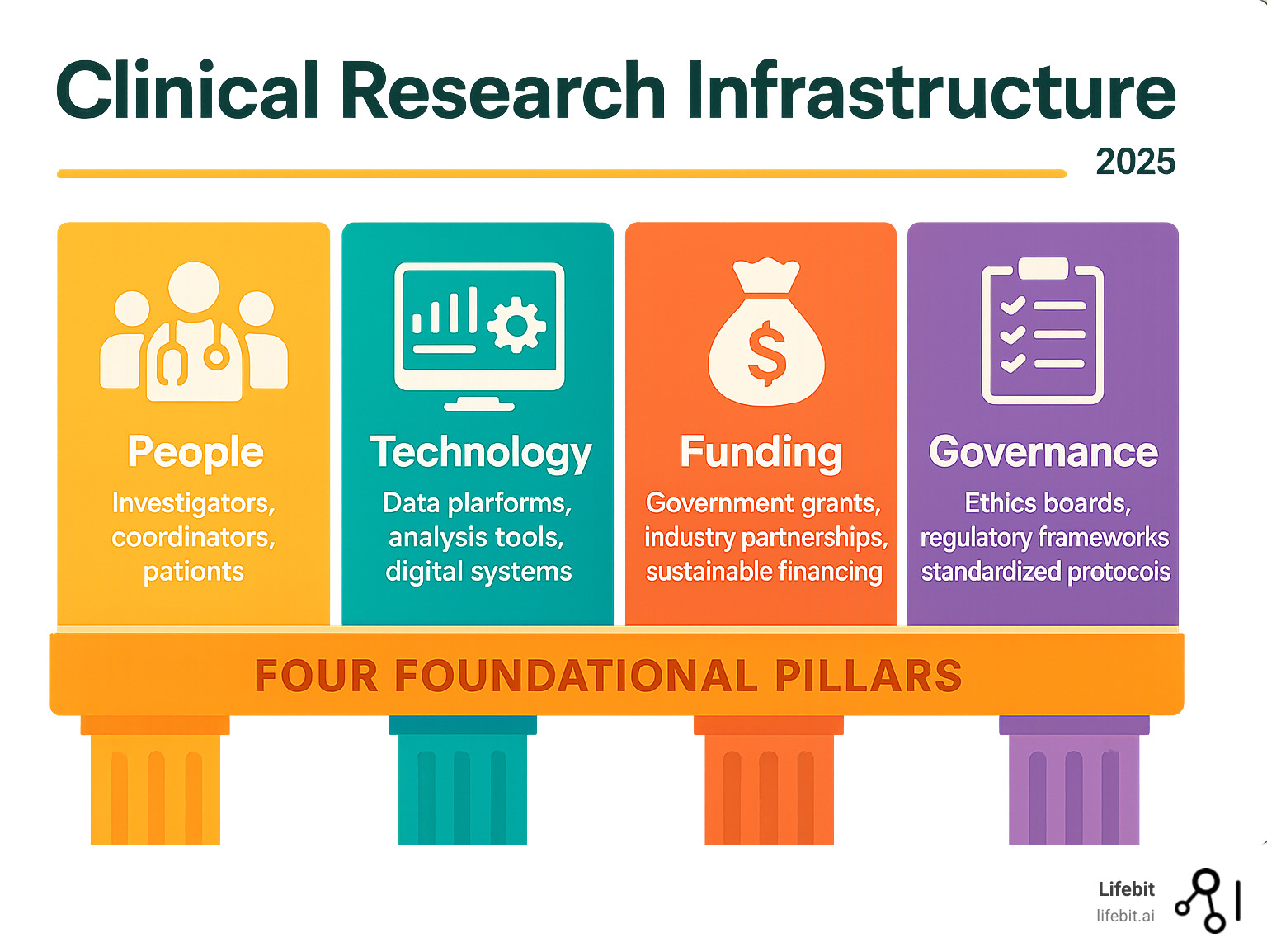
The Core Components of a Successful Clinical Research Infrastructure
Building a robust clinical research infrastructure requires three core components: talented people, solid processes, and the right tools. Remove any one, and the whole structure fails.

These pillars must work in harmony, like an orchestra: the musicians (human capital), the conductor and sheet music (operational frameworks), and the instruments and hall (physical and digital foundations).
Human Capital: Training and Engagement
People are the heart of any research program. Without skilled investigator training and genuine patient engagement, even the best equipment won’t produce meaningful results.
Clinician scientists face a unique challenge, needing to master both patient care and rigorous research. This means understanding everything from Good Clinical Practice (GCP) guidelines to complex statistical methods, which is why comprehensive training programs are essential.
Patient advocacy groups and community engagement aren’t just nice-to-have additions; they’re game-changers. When patients help shape research questions from the start, trials become more relevant, recruitment becomes easier, and results address real-world needs.
The University of Utah AcA program shows how this should work. Since 2009, it has supported 820 students in hands-on clinical research. The program’s commitment to diversity is special: 17.5% of participants are under-represented in medicine (URiM) students, exceeding the university’s overall undergraduate URiM enrollment. These students have contributed to 57 publications, proving that investing in diverse talent pays real dividends. Scientific research on developing future clinician scientists provides deeper insights into how these programs strengthen our research workforce.
Operational and Governance Frameworks
Even brilliant researchers need clear rules. Solid operational and governance frameworks ensure ethical oversight, data quality, and research transparency.
Centralized IRBs have revolutionized multi-site studies. Instead of each institution reviewing the same protocol, a single review streamlines the process. This approach dramatically reduces startup times and creates more harmonized regulations across sites.
Standard Operating Procedures (SOPs), while seemingly bureaucratic, are liberating. By defining how to handle data collection, adverse event reporting, and quality control, they free research teams to focus on generating reliable evidence. These procedures work with GCP guidelines to protect participants and ensure credible data.
The goal isn’t just compliance. Strong governance frameworks build trust with participants, regulators, and the public. When people know that rigorous ethical oversight and quality standards are in place, they’re more willing to participate in research and trust the results.
Physical and Digital Foundations
This is the actual infrastructure that makes research possible. Clinical trial networks connect researchers across vast distances, enabling the large-scale studies needed to answer complex medical questions.
Canada’s investment in academic medical centers through initiatives like the Accelerating Clinical Trials (ACT) consortium shows how national coordination can strengthen these networks. With 232 researchers working together across the country, they’re proving that collaboration beats competition.
Biocontainment facilities gained attention during the pandemic for good reason. These specialized labs allow researchers to safely study dangerous pathogens. Canada’s $127 million investment in upgrading these facilities through the Biosciences Research Infrastructure Fund (BRIF) demonstrates their critical importance.
But the real revolution is digital. Electronic Health Records (EHR) integration turns routine patient care into research opportunities. Researchers can identify potential candidates from existing medical records instead of starting from scratch. Real-world data (RWD) platforms take this further, showing how treatments perform in everyday clinical practice.
The essential digital tools that power modern research include Electronic Data Capture (EDC) systems for accurate data collection, Clinical Trial Management Systems (CTMS) for coordinating operations, eConsent platforms for accessible informed consent, and secure data analysis environments that protect sensitive information.
Data warehouses serve as the memory banks of modern research, storing vast amounts of information. When combined with federated analytics platforms, researchers can collaborate on sensitive data without compromising privacy—a crucial capability in our interconnected research landscape.
Global and National Strategies for Bolstering Research
Building world-class clinical research infrastructure requires global cooperation, as the challenges are too great for any single country. Governments worldwide are making serious investments, recognizing that strong research infrastructure is essential for public health, economic growth, and pandemic readiness.
The WHO’s Global Action Plan
The World Health Organization knows that strengthening clinical research infrastructure requires a collective effort. That’s what happened when they brought together researchers, funders, ethicists, regulators, and industry leaders in Geneva on June 18-19, 2024.
The WHO stakeholder consultation had a clear mission: to implement its ambitious resolution on clinical trials (WHA75.8) during 2024-2025. Representatives from countries like Canada, India, Nepal, and South Africa shared their challenges and successes, painting a real picture of what different nations need to strengthen their research capabilities.
The discussions revealed a commitment to global collaboration and resource optimization, but also that many countries are duplicating efforts. The consultation highlighted the urgent need to share tools, knowledge, and best practices more effectively.
The WHO’s approach is refreshingly inclusive. They’re not just talking to researchers in ivory towers; they’re planning additional consultations with patient groups and community representatives. Clinical trials only work when the people they’re meant to help trust and participate in them. You can dive deeper into the specifics in the WHO resolution on strengthening clinical trials.
National Investment: The Canadian Model
If you want to see what serious national investment in clinical research looks like, Canada is writing the playbook. The Canadian government put its money where its mouth was with a massive $250 million Clinical Trials Fund.
This fund is part of Canada’s even bigger $2.2 billion Biomanufacturing and Life Sciences Strategy. The thinking is smart: invest now in research capabilities, and you’ll be ready for the next pandemic while building a thriving life sciences sector that drives innovation.
The beauty of Canada’s approach is its structured investment across three strategic areas. The Accelerating Clinical Trials consortium brings together an impressive 232 researchers from coast to coast, turning scattered efforts into a powerful, coordinated network.
But Canada didn’t stop there. They’re also funding seven different training platforms because they know that great infrastructure means nothing without skilled people. These programs are designed to train the next generation of clinical scientists and researchers.
The Biosciences Research Infrastructure Fund adds another layer of serious investment, with over $127 million going toward upgrading biocontainment facilities. These specialized labs are critical for safely researching dangerous pathogens. The pandemic taught us that having world-class biocontainment capabilities isn’t optional—it’s essential for national security and public health.
What makes Canada’s model compelling is how it addresses multiple needs simultaneously: building pandemic preparedness, supporting biomanufacturing, fostering public-private partnerships, and creating a sustainable ecosystem for long-term growth. It’s a comprehensive approach that other nations are watching. You can explore more about Canada’s strategy and see how they’re turning investment into results.
Overcoming Challenges with Innovative Approaches
Despite significant investments, the road to medical progress is often bumpy. Persistent challenges in clinical research infrastructure, from fragmented systems to the sheer cost of trials, create opportunities for innovation. Indeed, some solutions emerging today would have seemed like science fiction a decade ago.

Addressing Gaps in the Modern Clinical Research Infrastructure
Despite progress, our current clinical research infrastructure has significant gaps that slow medical breakthroughs.
Fragmented systems are a major headache. Data lives in separate silos, systems don’t communicate, and valuable time is wasted piecing together information instead of analyzing it.
The high costs of clinical trials can be staggering, sometimes billions of dollars for a single study. This creates a real barrier, especially for smaller organizations or researchers studying rare diseases.
Enrollment difficulties plague almost every trial. Finding enough eligible patients is a major hurdle, and many promising studies fail simply because they can’t recruit enough participants. This problem is compounded by the lack of diversity in trials—if study participants don’t represent the real world, how can we be sure treatments will work for everyone?
The bureaucratic maze of inefficient implementation—slow regulatory processes, paperwork, and inconsistent procedures—can turn streamlined research into a frustrating obstacle course.
These regulatory problems aren’t just annoying; they actively hold back medical progress. When researchers spend months navigating different approval processes, patients ultimately pay the price through delayed access to potentially life-saving treatments.
Innovative Technologies Optimizing Trial Operations
Technology is stepping up to solve these challenges in ways.
Point-of-care randomization is changing patient recruitment. A doctor can see a patient who might be a fit for a trial and, with a few clicks in their electronic health record system, enroll them right in the clinic. It’s research integrated seamlessly into medical care.
Federated analytics solves the data privacy puzzle. Instead of moving sensitive patient data, which creates privacy risks, we send analysis tools to where the data lives. Algorithms run locally, and only anonymized results are shared. It’s a brilliant and secure method.
eSource data collection eliminates tedious and error-prone manual data entry. When information is captured digitally at the source, we cut out transcription errors and get cleaner, more reliable data.
Wearable technology brings trials into patients’ real lives. Instead of relying on patient memory during clinic visits, we can continuously monitor heart rates, sleep patterns, and activity levels in their natural environments. This provides a richer, more accurate picture of how treatments affect daily life.
AI-driven patient matching uses artificial intelligence to scan massive databases and identify patients who meet specific trial criteria, finding matches in seconds rather than months.
Finally, virtual data warehouses create secure, centralized hubs where authorized researchers can access trial data while maintaining strict privacy controls. This breaks down frustrating data silos and enables the collaborative research that leads to real breakthroughs.
These technologies aren’t just making trials faster and cheaper—they’re making them more inclusive and reflective of the real world, which means better treatments for everyone.
The Impact of Robust Infrastructure on Health Equity and Outcomes
Investing in robust clinical research infrastructure isn’t just about labs and equipment; it’s about creating pathways to better health for everyone. The effects touch every aspect of healthcare, from treatment development to patient access.
Think about personalized medicine. With strong infrastructure, researchers can analyze diverse patient data more effectively, uncovering how treatments work differently based on an individual’s genetics, background, or lifestyle. This means moving away from a “one pill fits all” approach to healthcare. Instead, doctors can tailor treatments to individual patients, leading to better outcomes and fewer side effects.
A key benefit is faster drug approval. Smooth trials with high-quality data and efficient processes bring promising treatments to patients months or even years sooner. For someone with a serious illness, that time difference can be everything. The streamlined processes in well-funded programs like Canada’s Clinical Trials Fund show how proper infrastructure investment directly translates to quicker access to therapies.
Improved patient safety is another crucial outcome. Better data collection and real-time monitoring help researchers spot potential problems much faster. Our platform at Lifebit, for instance, supports AI-driven safety surveillance that analyzes patterns across large datasets to identify adverse effects early. This leads to safer treatments and more confident patients.
But perhaps the most important impact is on health equity. Building inclusive research infrastructure addresses a fundamental problem in medicine: too many treatments have been developed and tested primarily on narrow patient populations.
Addressing health disparities happens when we deliberately design research systems to be more inclusive. This means funding trials in diverse communities, supporting researchers from underrepresented backgrounds (like the University of Utah’s AcA program does), and building trust with communities historically excluded from research.
The result is inclusive research that generates better evidence for everyone. When clinical trials include people of different ethnicities, ages, and backgrounds, the results are more reliable for the entire population. This creates a virtuous cycle: better evidence leads to more effective treatments and improved health outcomes across all communities.
At its core, robust clinical research infrastructure is about democratizing medical progress. It ensures that breakthrough treatments can benefit everyone who needs them, not just a privileged few.
Frequently Asked Questions about Clinical Research Infrastructure
Understanding the key concepts of clinical research infrastructure can feel overwhelming, but it’s crucial for anyone in medical research. Here are answers to some common questions.
What are the most critical components of a sustainable clinical research enterprise?
A sustainable research enterprise requires a combination of trained investigators, engaged patient communities, and robust data management and regulatory systems. The technical backbone is equally important. Secure digital platforms—like our federated AI platform at Lifebit—allow researchers to access and analyze data while maintaining the highest privacy standards.
However, none of this works without consistent funding from both public and private sources. Research is expensive, and sustainability means having reliable long-term financial support.
What makes an enterprise future-proof is its ability to adapt. The infrastructure must integrate new technologies like AI and federated analytics while also being designed for long-term data preservation. We’re not just building for today’s trials; we’re building for findies that haven’t been imagined yet.
How does collaboration improve clinical research outcomes?
Collaboration is where the magic happens in research. When researchers, funders, regulators, industry, and patient groups work together, data silos start breaking down. Regulatory processes that used to take forever become streamlined because everyone is on the same page.
Pooling resources allows researchers to conduct larger, more comprehensive trials that would be impossible for a single organization. And when patient groups are involved from the start, the research addresses real-world needs instead of just academic curiosities.
The speed difference is remarkable. Instead of researchers working in isolation and duplicating efforts, collaboration accelerates the entire process. It’s like switching from dial-up to fiber optic—suddenly, the impossible becomes efficient.
How does investing in research infrastructure address health disparities?
This is a critical question, and the answer offers real hope for the future of medicine. Investing in research infrastructure isn’t just about building better labs; it’s about building a more equitable healthcare system.
When we fund trials in diverse communities, we ensure that research reflects the real world. For too long, medical studies focused on certain populations, meaning treatments might not work as well for everyone else. By deliberately investing in infrastructure that reaches underserved areas, we start fixing this problem.
Training also matters enormously. Programs like the University of Utah’s Academic Associate initiative show what’s possible when we support the training of underrepresented researchers. When the people conducting research reflect the diversity of the patients they’re studying, the questions and methods become more inclusive.
But the key is building trust with underserved populations, which takes time and genuine commitment. It means creating infrastructure that is accessible, culturally sensitive, and transparent. When communities see that researchers are invested in their wellbeing, participation increases.
The end result is more inclusive trial data that ensures new treatments work safely and effectively for everyone. This is essential for creating treatments that improve global health outcomes. When we build clinical research infrastructure with equity in mind, we create a foundation for findies that benefit all of humanity.
Conclusion
The future of medicine is being written by clinical research infrastructure. Every trained investigator, secure data platform, and collaborative network we build today shapes the treatments that will save lives tomorrow.
We’re living through an exciting change. Technology-driven research is revolutionizing how we find new treatments, while global initiatives are ensuring these breakthroughs reach everyone, not just a privileged few. The pandemic taught us a hard lesson: countries with strong research networks could rapidly test and deploy life-saving interventions, while others watched from the sidelines.
This reality has sparked a new understanding of global health security. It’s no longer enough to have great hospitals or brilliant doctors in isolation. We need interconnected systems that can share data securely, collaborate across borders, and respond quickly to emerging health threats. The WHO’s recent stakeholder consultations and Canada’s massive infrastructure investments show that governments finally get it – clinical research infrastructure isn’t just nice to have, it’s essential for national security.
At Lifebit, we’re honored to be part of this critical mission. Our federated AI platform tackles one of the biggest challenges in modern research: how to analyze sensitive health data from around the world without compromising privacy. Our answer lies in bringing the analysis to the data, rather than the other way around.
Through our Trusted Research Environment, Trusted Data Lakehouse, and R.E.A.L. platform components, we’re enabling real-time insights from global datasets while maintaining the highest standards of data protection. When a researcher in Toronto can securely analyze data from London, Tokyo, and São Paulo without any patient information ever leaving its home country, we know we’re building the infrastructure for tomorrow’s medical breakthroughs.
The road ahead is challenging but promising. As we strengthen our clinical research infrastructure, we’re not just advancing science; we’re building a world where geography, economics, and politics don’t stand between patients and the treatments they need.
Find how to accelerate your research with next-generation infrastructure.

