Why Clinical Research Matters More Than You Think

Clinical Research: Unlocking 1 Healthier Future
Why Clinical Research is the Foundation of Every Medical Treatment You’ll Ever Receive
Clinical research is the systematic study of new treatments, medications, and medical devices in human volunteers to determine their safety and effectiveness. It is the foundational process that proves a medical treatment works before it reaches patients. Every drug, device, diagnostic test, and medical technique used today was first validated through clinical research. Without it, we wouldn’t have vaccines, cancer treatments, or even basic mammograms.
Quick Overview: What Clinical Research Involves
- Purpose: Test safety and effectiveness of new treatments
- Participants: Human volunteers who meet specific criteria
- Types: Observational studies and clinical trials
- Phases: Four phases from initial safety testing to long-term monitoring
- Protection: Strict ethical guidelines and oversight committees
- Impact: Every approved treatment was tested this way
The ClinicalTrials.gov database alone contains hundreds of thousands of studies, requiring people of every age, health status, and background to participate. While your doctor uses proven treatments (clinical practice), clinical research is the process of creating and validating those treatments.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent over 15 years changing how clinical research data gets analyzed across secure, federated environments. My background spans computational biology, AI, and health-tech entrepreneurship, giving me a front-row seat to how technology is revolutionizing medical research and drug findy.
Clinical research terms to learn:
The Foundation of Modern Medicine
Every medical intervention, from antibiotics to MRI scans, exists because of clinical research. This rigorous scientific process transforms promising ideas into proven treatments. However, this evidence-based approach is a relatively recent development in human history. For centuries, medical treatments were based on tradition, anecdote, and trial and error, often with little understanding of their true effects. The shift towards systematic investigation has been one of the most significant achievements in healthcare.
It’s crucial to distinguish between clinical practice—a doctor treating you with established, approved methods—and clinical research, the formal investigation that proves those methods are safe and effective in the first place. Before any medication is prescribed, it undergoes years of testing in clinical research studies involving thousands of volunteers. This process generates the evidence needed for regulatory bodies like the U.S. Food and Drug Administration (FDA) to grant approval. Without this foundation, modern medicine would be based on guesswork, not science.
A Brief History: From Anecdote to Evidence
The journey to modern clinical research was long and marked by key turning points that established the scientific and ethical standards we rely on today.
- Early Trials: One of the first recorded clinical trials was conducted in 1747 by James Lind, a ship’s surgeon. He systematically tested six different treatments for scurvy on 12 sailors, discovering that citrus fruits were a cure. His work demonstrated the power of a controlled experiment, a stark contrast to the unsubstantiated theories of the time.
- The Rise of Regulation: Despite early examples, the 19th and early 20th centuries saw the rise of the scientific method but lacked formal ethical oversight. This led to numerous instances of exploitation.
- The Nuremberg Code (1947): Following horrific experiments conducted during World War II, the Nuremberg trials produced a landmark document that established 10 principles for ethical human research. Its first and most important principle is that the voluntary, informed consent of the human subject is absolutely essential.
- The Declaration of Helsinki (1964): Developed by the World Medical Association, this declaration built upon the Nuremberg Code, providing a more detailed set of ethical principles for the medical community. It has been revised multiple times and serves as a cornerstone of international research ethics, emphasizing that the well-being of the research participant must always take precedence over the interests of science and society.
These historical milestones created the framework for the rigorous, ethically-governed process we know today.
| Feature | Clinical Practice | Clinical Research |
|---|---|---|
| Primary Goal | Provide individualized patient care using proven treatments. | Generate new, generalizable medical knowledge to prove the safety and efficacy of new interventions. |
| Interventions | Uses established, approved treatments with known risk-benefit profiles. | Tests investigational treatments or methods where the full risk-benefit profile is not yet known. |
| Focus | The well-being and health outcome of the individual patient. | Groups of patients to draw statistical conclusions that can be applied to future patients. |
| Methodology | Follows standard medical protocols and guidelines. | Follows a rigid, predefined study protocol with specific objectives, endpoints, and statistical plans. |
| Oversight | Medical licensing boards, hospital policies, and professional standards. | Institutional Review Boards (IRBs), regulatory agencies (e.g., FDA), and Data Safety Monitoring Boards (DSMBs). |
| Outcome | Improved health for the individual patient. | New evidence that informs future clinical practice and may lead to new approved treatments. |
More about the role of data in medical breakthroughs.
What is Clinical Research?
Clinical research is a systematic investigation involving human participants designed to answer specific questions about health and illness. Unlike basic science research conducted in a laboratory, it directly studies how interventions—such as new medications, medical devices, surgical procedures, and behavioral treatments—work in people. Every study follows a strict, predefined plan called a protocol and uses rigorous statistical analysis to ensure the conclusions are reliable. This process ensures that by the time a treatment reaches the public, we have solid evidence that its benefits outweigh its risks.
What Are the Different Types of Clinical Research?.
Why is it the Cornerstone of Healthcare?
Clinical research is the bedrock of evidence-based medicine. It provides the objective proof that underpins every prescription, procedure, and public health recommendation.
- Proving Safety: The primary and most critical goal is to demonstrate that a new treatment’s potential benefits outweigh its risks. Research systematically identifies and quantifies potential side effects, from common and mild to rare and serious.
- Proving Efficacy: Beyond safety, research must show that a treatment is effective for its intended purpose. It must produce a measurable, positive health outcome under controlled conditions.
- Advancing Patient Care: This continuous cycle of hypothesis, testing, and validation leads to better treatments, more accurate diagnostics, and effective prevention strategies, improving and extending lives globally.
- Understanding Disease: Research not only tests treatments but also helps us understand how diseases develop, progress, and affect different populations. This knowledge is crucial for developing targeted interventions and public health policies.
The Blueprint of a Study: Types and Phases of Clinical Research
Every clinical study follows a detailed blueprint called a protocol. This document is the instruction manual for the research, meticulously outlining the study’s objectives, methodology, participant criteria, data collection procedures, and safety measures. It ensures that the research is conducted consistently across all sites, is scientifically sound, and prioritizes the safety of participants. Without this meticulous plan, medical research would be unreliable and chaotic.
Types of Research: Observation vs. Intervention
Clinical research generally falls into two main categories, each answering different kinds of questions.
Observational Studies: Gathering Clues Without Intervening
In these studies, researchers observe participants to learn about health and disease without assigning a specific intervention. They are crucial for identifying risk factors and understanding disease patterns.
- Cohort Studies: Researchers follow a group of people (a cohort) over time, often for years or decades, to see how certain factors (like diet, exercise, or exposure to a substance) affect their health outcomes. The Framingham Heart Study, which began in 1948, is a famous example that identified major risk factors for cardiovascular disease.
- Case-Control Studies: These studies are retrospective. Researchers identify a group of people with a specific condition (cases) and compare them to a similar group without the condition (controls). They then look back in time to identify past exposures or factors that may be linked to the disease. This design was instrumental in establishing the link between smoking and lung cancer.
- Cross-Sectional Studies: This type of study provides a snapshot of a population at a single point in time. Researchers collect data on a group of people to assess the prevalence of a condition and its associated factors simultaneously. For example, a study might survey a community to determine the current rates of diabetes and its correlation with obesity.
Other types include biospecimen studies, which analyze samples like blood or tissue to find biomarkers, and healthy volunteer studies, which establish normal health baselines to help interpret data from patients.
Clinical Trials (Interventional Studies): Testing New Treatments
This is where researchers actively test a new intervention—such as a medication, device, or behavioral therapy—to evaluate its safety and effectiveness. Clinical trials provide the strongest form of evidence and are required before a new treatment can be approved for public use.
More on designing successful trials.
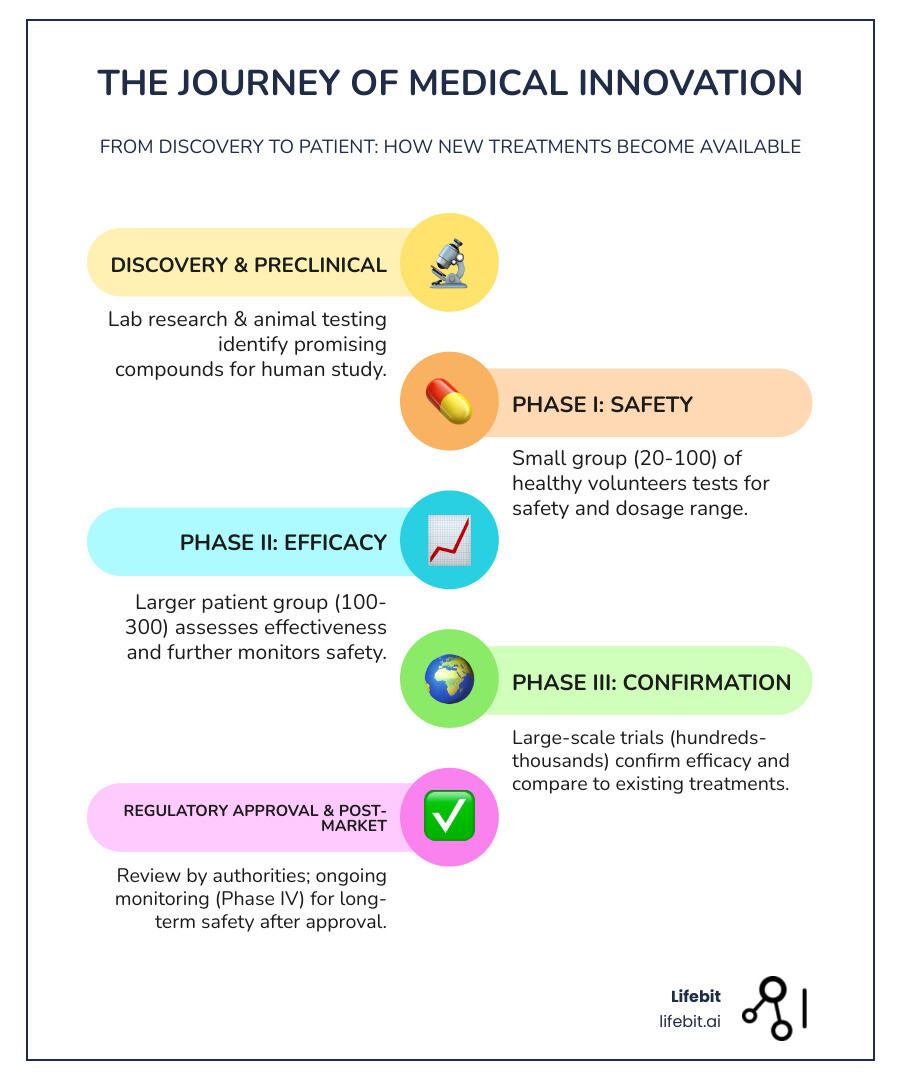
The Four Phases of a Clinical Trial: A Step-by-Step Journey
New treatments are tested through a careful, multi-phase approach to maximize safety and gather robust evidence. Each phase answers a different set of questions.
Phase I: Is it Safe?
This is the first time a new treatment is tested in humans. The primary goal is safety and dosage. In a small group (typically 20-100 healthy volunteers or patients with advanced disease), researchers evaluate how the treatment is absorbed and processed by the body (pharmacokinetics) and identify common side effects. They conduct dose-escalation studies to determine the maximum tolerated dose (MTD) before significant toxicity occurs. These studies usually last several months.
Phase II: Does it Work?
Once a safe dose is established, the treatment moves to Phase II to evaluate preliminary efficacy and side effects. With a larger group of patients who have the condition (100-300), the goal is to see if the treatment shows a therapeutic effect (proof of concept) while continuing to monitor safety. This phase helps refine the dosage for larger studies and has a high failure rate, as many treatments that are safe prove to be ineffective. This phase can last from several months to two years.
Phase III: Is it Better?
This phase is designed to confirm efficacy and compare the new treatment to existing standards. Involving a large, diverse population (1,000 to 3,000+ participants), these pivotal trials are typically randomized, controlled, and double-blinded to prevent bias. Participants are randomly assigned to receive either the new treatment or a control (a placebo or the current standard of care). The goal is to generate the definitive data on effectiveness and safety required for regulatory approval from agencies like the FDA. Successful completion, which can take one to four years, is the final hurdle before a treatment can be considered for market approval.
Phase IV: What Happens Long-Term?
Also known as post-market surveillance, this phase occurs after a treatment is approved and available to the public. It continues to study the treatment in thousands of patients to monitor long-term safety, real-world effectiveness, and identify rare side effects that may not have appeared in smaller Phase III trials. Phase IV studies can also explore new uses for the treatment. For example, aspirin was initially approved for pain relief, but later research demonstrated its value in preventing heart attacks, expanding its clinical use.
The People and Protections Behind the Science
Every clinical research study is a complex operation supported by a team of dedicated professionals and governed by strict international regulations. These rules, often summarized under the umbrella of Good Clinical Practice (GCP), are designed to ensure scientific integrity and, most importantly, participant safety. This robust ethical framework was not created in a vacuum; it was built on painful historical lessons, such as the unethical Tuskegee Syphilis Study and experiments conducted during World War II, which highlighted the absolute necessity of protecting human research subjects.

The Core Principles of Ethical Research
Modern research ethics in the United States are guided by the principles outlined in the Belmont Report (1979), which serves as the foundation for regulations protecting participants.
- Respect for Persons: This principle asserts that individuals are autonomous agents who must be able to make their own decisions. It is the basis for informed consent. It also calls for the protection of those with diminished autonomy (e.g., children or individuals with cognitive impairments).
- Beneficence: This principle involves two complementary duties: do no harm, and maximize possible benefits while minimizing possible harms. It requires researchers to carefully weigh the risks and benefits of every study.
- Justice: This principle addresses the fairness of who bears the burdens and who receives the benefits of research. It ensures that specific groups (e.g., vulnerable populations) are not unfairly selected for risky research and that all groups have an opportunity to participate in and benefit from scientific advances.
Key Roles and Responsibilities
A team of individuals with distinct responsibilities ensures each study runs smoothly, ethically, and safely:
- Sponsor: The organization (e.g., a pharmaceutical company, university, or government agency like the NIH) that initiates, funds, and oversees the study. The sponsor is responsible for the overall study design, data quality, and reporting results to regulatory authorities.
- Principal Investigator (PI): A qualified physician or researcher who leads the study at a specific research site. The PI is directly responsible for conducting the study according to the protocol and for protecting the rights, safety, and welfare of participants at their site.
- Clinical Research Coordinator (CRC): Manages the daily operations of the study, from screening and enrolling participants to collecting data, managing schedules, and serving as a primary point of contact for participants.
- Institutional Review Board (IRB): An independent committee composed of scientists, non-scientists, and community members. Its primary mission is to protect the rights and welfare of human research subjects. The IRB must review and approve all study-related materials—including the protocol, informed consent form, and recruitment advertisements—before a study can begin and must conduct ongoing reviews.
- Data and Safety Monitoring Board (DSMB): An independent group of experts that periodically reviews study data as it is collected. The DSMB monitors for any emerging safety concerns and can recommend that a trial be modified or stopped if participants are being exposed to unexpected or unacceptable risks.
- Study Participant: The volunteer at the very center of the research. Their safety, rights, and well-being are the top priority for the entire research team.
Ethical Safeguards for Participants
Strong, enforceable protections are in place for every research volunteer.
- Informed Consent: The Cornerstone of Protection: This is a process, not just a document. Before joining, a potential participant must receive a thorough explanation of the study and have an opportunity to ask questions. The signed consent form is a record of this discussion, and it must clearly detail:
- The study’s purpose, expected duration, and all procedures involved.
- Any foreseeable risks, discomforts, or side effects.
- Any potential benefits to the participant or to others.
- Available alternative treatments or procedures.
- The extent to which confidentiality of records will be maintained.
- Information on compensation or medical treatment available in case of injury.
- A clear statement that participation is voluntary and that the participant can withdraw at any time without penalty.
- Voluntary Participation: Participation is always a choice. A volunteer can decline to join or can leave a study at any time, for any reason, without it affecting their standard medical care.
- Confidentiality: Strict privacy rules, such as those under HIPAA in the U.S., protect participants’ personal health information. Data is typically de-identified and coded to ensure privacy is maintained throughout the study and in any publications.
- Risk-Benefit Ratio: The IRB must determine that the potential benefits of a study justify the potential risks. Researchers are ethically bound to design studies that minimize all potential risks to participants.
Many institutions also have Research Participant Advocacy Groups to provide independent support and resources for volunteers.
Research Participant Advocacy Group.
Ensuring GDPR compliant data.
The Power of Data and the Future of Clinical Research
Data is the lifeblood of clinical research. It is the raw material that, when collected and analyzed correctly, becomes the evidence that guides medical breakthroughs, regulatory approvals, and everyday patient care. Today, a technological revolution driven by AI, genomics, and new data collection methods is rapidly expanding the boundaries of what’s possible in healthcare, making research more powerful and precise than ever before.

The Role of Data and Evidence in Clinical Research
Every study is designed to answer specific questions by measuring predefined outcomes, or endpoints. These are the metrics used to judge a treatment’s success.
- Primary Endpoint: This is the main outcome the study is designed to measure and the one on which its success is judged. It must be a clinically meaningful measure that directly answers the primary research question. For example, in a cancer trial, the primary endpoint might be “overall survival rate at five years.”
- Secondary Endpoints: These are additional important outcomes that provide supportive information about a treatment’s effects. Examples include quality of life scores, time until disease progression, or the rate of certain side effects.
- Exploratory Endpoints: These are often measured to generate new hypotheses for future research. For instance, a study might measure levels of a novel biomarker to see if it correlates with how well patients respond to the treatment.
The data landscape has expanded beyond traditional trials to include Real-World Data (RWD). This refers to health data collected outside of clinical trials, from sources like electronic health records (EHRs), insurance claims, patient registries, and wearable devices. When analyzed, RWD generates Real-World Evidence (RWE) on how treatments perform in everyday clinical practice. RWE is invaluable for understanding long-term safety, comparing the effectiveness of different treatments, and identifying unmet needs in diverse patient populations.
Making sense of this data deluge is a major challenge. At Lifebit, our federated AI platform enables secure access to global biomedical data, helping researchers generate reliable insights from vast, diverse datasets while maintaining patient privacy and data security.
Benefits of Real-World Data in Clinical Research.
The Future of Clinical Research: AI, Genomics, and Decentralisation
The future of clinical research is being reshaped by powerful technological forces that promise to make studies faster, smarter, and more patient-centric.
- Artificial Intelligence (AI) is acting as a powerful research assistant. It accelerates drug discovery by identifying novel biological targets, optimizes trial design by simulating outcomes, and improves patient recruitment by scanning EHRs to find eligible participants. In data analysis, AI can detect complex patterns in imaging, genomic, or RWD that are invisible to the human eye, helping to predict patient responses.
- Genomics and Precision Medicine: Genomics allows researchers to analyze a person’s genetic code to understand their disease at a molecular level. This enables the development of targeted therapies that are more effective for specific patient subgroups. This approach often involves a companion diagnostic, a test required to see if a patient has the right biomarker (e.g., a genetic mutation) to benefit from a particular drug, such as HER2 testing for Herceptin in breast cancer.
- Digital Biomarkers: Data from smartwatches, phones, and other sensors provide a continuous, real-time picture of a patient’s health (e.g., activity levels, sleep patterns, heart rate). This offers richer, more objective insights into a treatment’s effect on daily life compared to periodic clinic visits.
- Decentralized Clinical Trials (DCTs) represent a major shift in how research is conducted. By using telemedicine, home health visits, wearable sensors, and direct-to-patient drug delivery, DCTs bring the trial to the participant.
- Benefits: This model makes trials more accessible to patients in remote or underserved communities, increases participant diversity, improves retention by reducing the travel burden, and allows for more continuous data collection.
- Challenges: Key hurdles include the “digital divide” (inequitable access to technology), ensuring the quality and security of remotely collected data, and navigating evolving regulatory frameworks for this new model.
Our Federated Data Analysis approach is designed to power these advances. It allows researchers to analyze sensitive data across different institutions and countries without the data ever leaving its secure environment. This enables global collaboration on an unprecedented scale while ensuring top-tier privacy and security, accelerating the path from discovery to cure.
Genomics.
Federated Data Analysis.
AI in Drug Development.
Should You Participate? A Guide for Volunteers
Deciding to join a clinical research study is a personal choice. Millions participate each year, some as healthy volunteers and others as patients seeking new options. By participating, you become a partner in the scientific process, helping bridge the gap between today’s medical limitations and tomorrow’s breakthroughs.
Finding and Evaluating a Clinical Trial
Finding the right study starts with knowing where to look and what to ask.
- Where to Look: ClinicalTrials.gov is a comprehensive database of studies worldwide. Your doctor, local hospitals, and patient advocacy groups are also excellent resources.
- Eligibility Criteria: Every study has inclusion criteria (requirements to join) and exclusion criteria (factors that prevent participation). These rules ensure participant safety and the reliability of the study results. A research team will walk you through these requirements.
- Ask Questions: The informed consent process is designed for you to get answers. Ask about the number of visits, potential side effects, time commitment, and what happens if you decide to leave the study.
Learn about studies at ClinicalTrials.gov.
Innovations in clinical trial recruitment.
Potential Benefits and Risks of Participation
Participating in clinical research has both potential benefits and risks to consider.
Potential benefits can be significant. You may gain access to new treatments not yet publicly available and receive expert medical care with close monitoring, often at no cost. Many also find deep satisfaction in contributing to science and helping future patients. Some studies offer financial compensation for time and travel.
Potential risks must also be considered. The investigational treatment may cause unexpected side effects or prove to be ineffective. Participation also requires a time commitment for visits and procedures, which can disrupt your daily routine.
For patients with serious conditions who cannot join a trial, Expanded Access (or compassionate use) may be an option. This program provides access to experimental treatments outside of a formal trial, though it is not available for every treatment.
Honest conversations with the research team about your expectations and concerns are key to making an informed decision.
Frequently Asked Questions about Clinical Research
Here are answers to some of the most common questions about clinical research.
What is the difference between a blind and an open-label study?
In a blind study, participants (and sometimes researchers in a “double-blind” study) don’t know who is receiving the experimental treatment versus a placebo or standard care. This method prevents bias, as expectations can influence outcomes. In an open-label study, everyone involved knows what treatment is being administered. These are used when blinding is not practical or ethical.
Do I get paid to participate in a clinical trial?
It depends on the study. Some clinical research studies offer compensation to reimburse participants for their time and travel expenses, while others do not. Any compensation will be clearly detailed in the informed consent document. The goal is reimbursement, not to pressure anyone into joining.
Can I leave a clinical trial after it has started?
Yes, absolutely. Participation in clinical research is always voluntary. You can withdraw from a study at any time, for any reason, without penalty. Your decision will not affect your regular medical care, and your well-being always comes first. The research team will respect your choice.
Conclusion: Powering the Next Generation of Medical Research
Clinical research is the engine of medical progress. Every prescription, diagnostic scan, and vaccine exists because volunteers participated in studies. Without this evidence-based approach, medicine would rely on guesswork, and progress would halt.
Today, we are witnessing a profound shift in how clinical research is conducted. The move toward data-driven, decentralised trials is rewriting the rules. Technologies like Artificial Intelligence, digital biomarkers, and genomics are enabling researchers to analyze more data, reach more diverse populations, and bring treatments to patients faster than ever before. The role of technology in accelerating research has never been more critical.
At Lifebit, we are helping to lead this revolution. Our federated platform enables secure, large-scale research by connecting data from around the world while keeping it protected and compliant. We believe that when researchers can safely collaborate with vast datasets, breakthroughs happen faster, safety is monitored more effectively, and new treatments reach patients sooner.
We are proud to power this next generation of medical research, working with partners who share our vision of a healthier world. Clinical research is about more than data; it’s about hope, healing, and the incredible progress we achieve when science and human generosity unite.

