Software Solutions That Make Decentralized Clinical Trials a Breeze

Decentralized Clinical Trials Software: Seamless 2025
Why Decentralized Clinical Trials Software is Changing Healthcare Research
Decentralized clinical trials software is revolutionizing medical research by bringing trials to patients’ homes. These platforms enable remote monitoring, digital consent, real-time data collection, and virtual coordination, making trials more accessible, efficient, and patient-centered.
Key capabilities include:
- Digital patient recruitment and AI-powered screening
- Interactive electronic consent (eConsent)
- Remote monitoring via wearables, sensors, and apps
- Virtual study visits through telehealth
- Real-time data capture from electronic patient-reported outcomes (ePRO)
- Direct-to-patient logistics for medication and samples
- Centralized data management with real-time analytics
The global decentralized clinical trials market was valued at $15.1 billion in 2022 and is projected to reach $57.9 billion by 2030, exhibiting a CAGR of 18.5%. This reflects a fundamental shift in clinical research.
Traditional trials face major barriers, with geographic constraints and rigid schedules excluding 97% of potential patients. DCT software changes this. As one expert noted, “Decentralized clinical trials are changing clinical research by delivering better patient experiences and accelerating drug development.” These platforms remove barriers, reduce patient burden, and enable diverse participation while maintaining data quality and regulatory compliance.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in computational biology, AI, and health-tech, we’ve seen how decentralized clinical trials software can open up the power of real-world evidence while upholding the highest standards of data security and compliance.
Basic decentralized clinical trials software vocab:
The Core Benefits of Adopting Decentralized Clinical Trial Software
Adopting decentralized clinical trials software fundamentally changes how we connect with patients and conduct research, creating a cascade of benefits that address the most persistent challenges in drug development. By moving the trial’s center of gravity from the clinic to the patient’s home, these platforms open up unprecedented gains in access, efficiency, and data richness.
The most striking change is in patient recruitment. Traditional trials are notoriously slow and struggle with diversity because they are limited by geography. For instance, while Black and Hispanic individuals represent approximately 13% and 18% of the U.S. population, their participation in clinical trials often falls into the single digits. DCT software shatters these geographic barriers, expanding the reach to patients who live far from major research sites, cannot take time off work, have mobility challenges, or are caregivers. This expanded reach is the cornerstone of building more diversity and inclusion into research, bringing in participants from rural and underrepresented communities. The result is a participant pool that more accurately reflects the real-world population, ensuring that new treatments are proven to be safe and effective for everyone.
With a broader, more accessible pool of participants, patient retention improves dramatically. Dropout rates in traditional trials can be as high as 30%, often due to the high “burden of participation”—frequent travel, long waits, and time away from family and work. DCT software reduces this burden by allowing patients to participate from the comfort of their homes. Features like automated reminders, easy-to-use apps, and telehealth check-ins make participation a seamless part of their daily lives, not a disruptive obligation. This convenience and respect for patients’ time are directly linked to higher engagement and retention rates, ensuring studies maintain statistical power and are completed on schedule.
Simultaneously, the data quality captured through DCT platforms is often superior to that from traditional methods. Instead of relying on episodic data points collected during infrequent site visits—which are subject to recall bias—DCT software captures high-frequency, real-time information through electronic patient-reported outcomes (ePROs) and connected wearables. This continuous stream of real-world data (RWD) provides a much clearer and more objective picture of a patient’s health status, treatment response, and quality of life. This allows researchers to detect subtle changes, identify safety signals earlier, and gain deeper insights into the patient experience.
These improvements culminate in greater trial efficiency and significant cost reduction. With the average cost of site start-up at $42,700 and per-patient costs running into the tens of thousands, the savings from reducing physical infrastructure, on-site monitoring visits, and patient travel reimbursements are substantial. Faster recruitment and better retention lead to accelerated timelines, which is the ultimate goal. Bringing a life-saving therapy to patients even a few months sooner can have an immense impact on public health and represents millions of dollars in revenue for sponsors, creating a powerful win-win scenario.
More info about the decentralized clinical trial model
Enhancing Patient Access and Engagement
The human side of research is where decentralized clinical trials software shines, directly addressing the logistical and personal barriers that exclude an estimated 97% of potential patients from participating in traditional studies.
The reduced travel burden is a game-changer. Consider a patient with a rare cancer living 150 miles from the nearest specialist research center. In a traditional model, participation would be nearly impossible. With DCT software, that patient can enroll online, conduct virtual visits via video calls, report outcomes on a mobile app, and have study medication delivered to their door. This eliminates the financial and physical strain of long trips and time off work. Modern platforms also provide real-time support through secure messaging or on-demand video calls with study coordinators, helping patients feel connected, supported, and confident in their participation.
The user-friendly interfaces of modern DCT platforms are critical for sustained engagement. Designed with the simplicity of familiar consumer apps, they make it easy for patients of all ages and technical abilities to steer tasks. This includes designing for accessibility, with features like adjustable text sizes and compatibility with screen readers. Simple yet powerful features like automated reminders for medication doses or survey completion help maintain protocol adherence without being intrusive, while gamification elements like progress trackers can further boost motivation and long-term engagement.
Watch Video One Intuitive User Experience
Innovations in Clinical Trial Recruitment and Enrollment
Improving Data and Operational Efficiency
Beyond the patient experience, decentralized clinical trials software revolutionizes the operational engine of clinical research, driving efficiency from data collection to regulatory oversight.
Real-time data capture means that information flows continuously from patients’ daily lives directly into secure study systems. This constant stream of data from ePROs, wearables, and home-based devices allows study teams to spot trends, identify potential safety signals, and make informed decisions much faster than in a traditional trial. The reduced errors from automated, digital data collection are also a major benefit. This eliminates the time-consuming, error-prone work of transcribing data from paper sources and the subsequent need for extensive data cleaning and reconciliation.
Streamlined workflows automate many of the repetitive, administrative tasks that bog down site coordinators, such as scheduling appointments, sending reminders, and tracking logistics. For example, an automated workflow could be configured so that a patient’s ePRO entry indicating a high pain score automatically triggers an alert to the study nurse for follow-up. This frees up site staff to focus on high-value activities like patient support and relationship-building. Furthermore, remote monitoring capabilities allow sponsors and CROs to oversee trial progress and data quality using real-time dashboards. This risk-based approach enables them to identify and address issues at specific sites or with individual patients immediately, rather than waiting weeks or months for an on-site visit to uncover a problem, saving both time and money while improving trial quality.
Unified Platform for All Stakeholders
Clinical Research SaaS Technology
Key Categories of Software Powering Modern DCTs

The decentralized clinical trials software landscape is a rich ecosystem of specialized tools working in harmony to support the entire research lifecycle. The industry is moving away from rigid, monolithic systems toward flexible technology stacks. Modern DCT technology reflects this, with organizations choosing between fully integrated platforms that provide a seamless, end-to-end solution or modular solutions that allow them to assemble a best-in-class toolkit for their specific needs. In either model, Open API capabilities are essential, ensuring that different systems—from eConsent to supply management—can communicate seamlessly. This comprehensive, interconnected approach transforms the entire research process, from initial protocol design to final data lock.
Trial Design and Start-Up
A solid foundation is critical for any trial, and decentralized clinical trials software helps build it with greater speed and intelligence.
Protocol development tools have evolved beyond simple document templates. Modern software can guide researchers through creating a study blueprint, offering libraries of standard endpoints and assessments. Advanced platforms even include simulation features that model different trial designs (e.g., fully decentralized vs. hybrid) to forecast recruitment timelines, patient burden, and budget impact. For site selection and site start-up, the software streamlines what was once a lengthy, paper-intensive process. It can analyze real-world data to identify geographic hot spots with high concentrations of eligible patients, helping sponsors select the right sites or even partner with local clinics and pharmacies. The software then acts as a central hub to manage contracts, track regulatory submissions, and monitor site activation status in real time. Moving to eTMF/eISF (electronic Trial Master File/investigator Site File) systems is a cornerstone of this efficiency. These systems make essential documents instantly and securely accessible for remote review by monitors, auditors, and regulators, eliminating shipping costs and delays while providing robust version control and audit trails.
Help with research protocol design
Modernizing Clinical Oversight
Patient Recruitment and Onboarding
Decentralized clinical trials software turns patient recruitment from a slow, manual effort into a precise, data-driven, and efficient process.
Digital recruitment platforms leverage a multi-channel approach, using targeted advertisements on social media, partnerships with online patient advocacy groups and health communities, and analysis of de-identified EHR data to find potential participants. This dramatically speeds up timelines and reaches a broader, more diverse audience. Once potential candidates are identified, remote pre-screening via online questionnaires or chatbots saves significant time for both patients and sites by ensuring only the most promising candidates proceed. eConsent is a major patient-friendly innovation. Instead of dense, legalistic paper documents, eConsent platforms use interactive multimedia—such as videos, animations, and diagrams—to explain the trial clearly and concisely. Embedded quizzes can test comprehension, ensuring patients make a truly informed decision at their own pace. This process is not only more engaging but also creates a more robust, auditable record of informed consent. Finally, built-in patient eligibility verification workflows guide site staff through the inclusion/exclusion criteria, ensuring everyone enrolled is appropriate for the study.
Digital Clinical Trial Recruitment
Informed Consent Best Practices
Patient Engagement and Data Collection
Decentralized clinical trials software transforms patient engagement and data collection into a continuous, seamless process that improves both the patient experience and the quality of the data.
ePRO and eCOA (electronic Patient-Reported Outcomes/Clinical Outcome Assessments) tools are central to this. They allow patients to log symptoms, quality of life, and other experiences in real-time on their own devices through intuitive apps. This method of “ecological momentary assessment” minimizes recall bias and provides a more accurate picture of the patient’s condition between site visits. The integration of wearables & sensors enables passive, objective data collection, capturing continuous physiological insights without requiring any effort from the patient. This can include data from smartwatches (heart rate, activity levels), ECG patches (cardiac rhythms), continuous glucose monitors, smart inhalers, and digital scales. Telehealth capabilities, including secure video conferencing and messaging, make it possible for anyone to participate in routine check-ins, regardless of their location. Finally, medication adherence can be tracked through smart pill bottles or patient self-reports, with automated reminders helping patients stay on schedule while giving study teams clear visibility into compliance.
BYOD vs. Provisioned Devices
A key consideration in DCT design is whether to use a Bring Your Own Device (BYOD) model, where patients use their own smartphones, or a provisioned device model, where the sponsor provides a pre-configured device. BYOD is convenient for patients and cost-effective but can introduce variability in hardware, operating systems, and data connectivity. Provisioned devices ensure a consistent user experience and data standard but add logistical complexity and cost. Modern DCT software is flexible enough to support either model, or a hybrid of the two, allowing sponsors to choose the best approach for their study population and data requirements.
Ensuring patients keep participating in the trial
Enhancing Clinical Trial Matching: Lifebit Patient Management
Trial Operations and Logistics
Decentralized clinical trials software is the command center that automates and orchestrates the complex logistics of running a modern trial.
Direct-to-Patient (DtP) shipping is a core component, sending study medication, sample collection kits, and connected devices directly to patients’ homes. This removes a major barrier to participation and improves convenience. The software manages the entire workflow, from inventory management and order fulfillment to tracking shipments and ensuring the chain of custody is maintained, which is especially critical for temperature-sensitive biologics. Remote monitoring gives sponsors and CROs real-time visibility into trial performance through centralized dashboards. This facilitates a risk-based quality management approach, where monitoring efforts are focused on the areas of highest risk, rather than conducting costly and inefficient 100% source data verification. Integrated supply management ensures that all necessary materials—from kits to devices—reach patients and sites on time. To reduce the administrative burden on research sites, site payments can be automated, with software triggering payments based on completed patient activities, improving cash flow and strengthening the sponsor-site relationship.
Home Health Integration
For hybrid trials that require procedures like blood draws or drug infusions, DCT software can integrate with networks of home health agencies. The platform can manage the scheduling and dispatch of nurses for in-home visits, provide them with the necessary protocols and instructions, and serve as the hub for collecting the data or samples from that visit, seamlessly blending in-person care with a remote trial framework.
DtP Fact Sheet
One Solution For Sites
Essential Features of a Modern DCT Platform

When choosing decentralized clinical trials software, the key is finding a unified platform with a comprehensive feature set that supports every stakeholder—patients, sites, sponsors, and CROs. A fragmented approach using multiple, disconnected point solutions often leads to data silos, integration headaches, and a confusing experience for users.
A truly comprehensive platform must natively integrate Electronic Data Capture (EDC) with electronic Patient-Reported Outcomes (ePRO) and electronic Clinical Outcome Assessments (eCOA). This creates a single source of truth, combining clinician-entered data with the patient’s real-world experience. Electronic consent (eConsent) should be a core, interactive module, not a bolt-on, making the process intuitive for patients to understand and simple for sites to administer and track.
Non-negotiable features include fully embedded telemedicine and virtual visit capabilities and robust Direct-to-Patient (DtP) logistics management, all managed within the same unified system. The platform must also be architected to seamlessly integrate wearable devices and sensors, allowing objective, high-frequency, and continuous data to flow directly and securely into the trial database without manual intervention.
The platform’s underlying architecture is equally important. True scalability ensures it can handle trials of any size and complexity, from a small Phase I study to a global Phase III program. Interoperability, powered by a robust API, allows it to connect with external systems like hospital EHRs and central labs. Above all, the user experience (UX) must be thoughtfully designed and intuitive for every user type. A simple, engaging app for patients is just as important as a powerful, efficient dashboard for study managers.
Data Management and Security in Decentralized Clinical Trial Software
With sensitive patient data being collected remotely and transmitted digitally, security and data integrity are paramount. Decentralized clinical trials software must be built on a foundation of trust, transparency, and robust protection.
Effective data integration is the first step, pulling information from disparate sources—apps, wearables, EHRs, and labs—into a single, coherent, and analyzable dataset. Real-time analytics dashboards then turn this raw data into actionable insights, allowing teams to monitor trial progress, patient safety, and data quality as events happen. AI and machine learning are supercharging this process. At Lifebit, our AI-powered pipelines can automatically process and harmonize complex genomic and clinical datasets. Machine learning models can be trained to predict patient dropout risk by analyzing engagement patterns (e.g., missed ePRO entries, frequency of logins), allowing for proactive intervention. AI can also power advanced safety surveillance, detecting potential adverse event patterns across thousands of patients far faster than human review.
Regulatory compliance with global data privacy standards like HIPAA in the U.S. and GDPR in Europe must be engineered into the platform’s core. This includes features for granular consent management and strict access controls that enforce the “minimum necessary” principle. All data must be protected with strong data encryption, both in transit over networks and at rest in the database. Robust, role-based access controls, comprehensive audit trails that log every action, and data pseudonymization are all critical components to ensure sensitive patient information remains secure and private throughout the trial lifecycle. Many advanced platforms achieve this through a Trusted Research Environment (TRE), a highly secure virtual space where data can be analyzed by approved researchers without being moved or downloaded, providing the highest level of protection.
What is a Secure Data Environment (SDE)?
Clinical Trial Success: Secure Data Platforms
Integration and Compliance Capabilities
The best decentralized clinical trials software does not exist in a vacuum; it connects seamlessly with the broader research and healthcare ecosystem.
EHR integration is critical for streamlining workflows and improving data quality. Using modern standards like HL7 FHIR (Fast Healthcare Interoperability Resources), the platform can pull patient demographic and medical history data directly from the hospital’s system, reducing manual data entry errors and enabling remote source data verification (SDV). API access provides the flexibility to connect with other best-in-class systems, such as specialized Laboratory Information Management Systems (LIMS) for sample tracking, imaging systems (PACS), or unique patient engagement tools, preventing vendor lock-in and allowing for a custom technology stack.
Compliance with FDA 21 CFR Part 11 is a fundamental requirement for trials conducted in the U.S. This regulation dictates the requirements for electronic records and signatures, ensuring they are as trustworthy as their paper equivalents. A compliant platform must include features like secure, role-based logins, tamper-evident, time-stamped audit trails for all data changes, and legally binding electronic signatures. As trials become more global, the platform must also be designed to support evolving global regulatory standards, such as those from the European Medicines Agency (EMA) and other national bodies, to ensure data can be collected, managed, and submitted compliantly across borders.
Facilitating Decentralised Clinical Trials in the EU – EMA
What Are Decentralized Clinical Trials? FAQ Sheet
Frequently Asked Questions about Decentralized Clinical Trial Software
Researchers, sponsors, and sites often have thoughtful questions about how decentralized clinical trials software can solve longstanding challenges. Here are the most common ones we encounter.
How does decentralized clinical trial software improve patient diversity?
This is one of its most impactful benefits. Traditional trials often have a diversity problem due to logistical barriers. By removing the need for patients to travel to specific research sites, decentralized clinical trials software opens participation to a much broader population. This includes patients in rural areas, individuals with mobility issues, and working people who cannot take time off for frequent visits. It also improves access for underrepresented ethnic and racial groups, addressing a persistent challenge in research and leading to treatments that are safer and more effective for everyone.
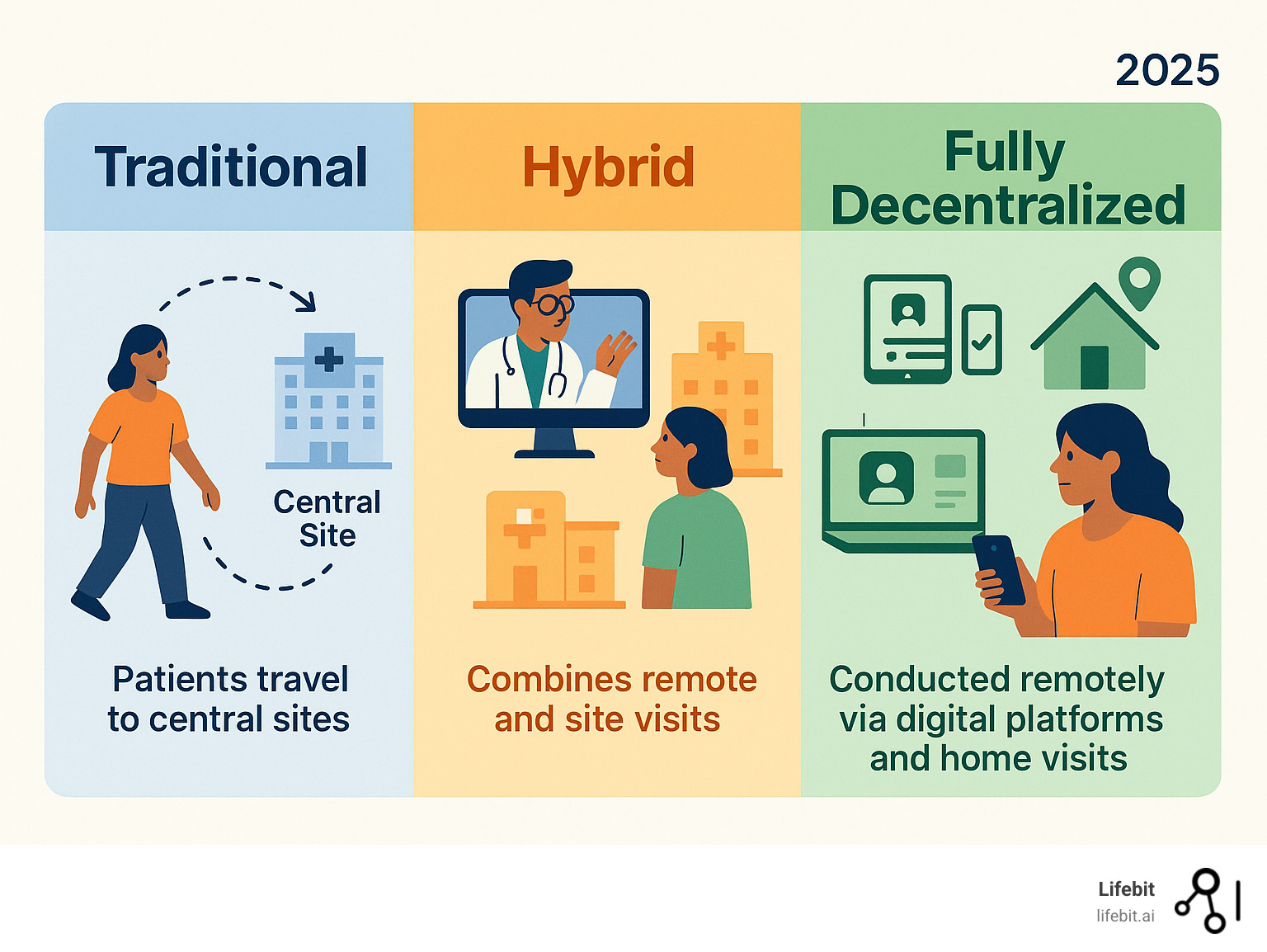
What is the difference between a hybrid and a fully decentralized trial?
Understanding this distinction is key to designing the right study. A fully decentralized trial conducts nearly all activities remotely. Patients are recruited online, provide eConsent on their devices, submit data via apps and wearables, and receive medication through direct-to-patient shipping. The entire process is managed by the software. A hybrid trial blends remote activities with traditional site-based visits. For example, a patient might visit a clinic for a complex procedure like an MRI but handle all routine check-ins and data reporting remotely. This model offers a flexible balance between scientific rigor and patient convenience.
How does AI improve DCT software?
Artificial intelligence makes DCTs smarter, safer, and more efficient across the entire trial lifecycle.
- Patient recruitment: AI can analyze health records (with appropriate consent and privacy controls) to identify eligible candidates, accelerating recruitment and improving diversity.
- Predictive analytics: Machine learning models can predict which patients are at risk of dropping out, allowing study teams to intervene proactively.
- Real-time safety surveillance: AI can continuously monitor incoming data to detect adverse event patterns much faster than manual reviews.
- Advanced analytics: AI and machine learning can uncover subtle insights from the massive, complex datasets generated by wearables and ePROs, open uping the full value of real-world data.
The Future is Federated: Choosing the Right Platform
The future of clinical research is here, with decentralized clinical trials software fundamentally changing how we develop new treatments. The market’s projected growth to $57.9 billion by 2030 confirms this is the new reality.
This future is powered by AI-driven insights. As we collect vast amounts of real-world evidence from wearables and ePROs, AI is the key to open uping its potential, helping us spot patterns, predict patient outcomes, and ensure safety in real-time. This technological shift is also reshaping roles, with sponsors, CROs, and sites evolving to manage data-driven, patient-centric trials.
The most exciting development is federated learning. This approach solves a major research challenge: how to analyze sensitive data from multiple sources without compromising privacy. Instead of moving data, federated learning brings the analysis to the data’s secure location.
This is where Lifebit is making a real difference. By leveraging next-generation federated platforms, organizations can securely access and analyze sensitive, distributed biomedical data without moving it, ensuring compliance and accelerating the delivery of new therapies. Our solutions, including the Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer), deliver real-time insights while keeping data secure.
This federated approach resolves the conflict between data access and security. It means faster findies, safer treatments, and better outcomes for patients worldwide. The future of clinical research isn’t just decentralized—it’s intelligent and federated.
Lifebit Trusted Research Environment
Explore the Lifebit Platform

