The Complete Guide to Digital Clinical Trial Recruitment

Why Digital Clinical Trial Recruitment is Critical for Modern Drug Development
Digital clinical trial recruitment is changing how pharmaceutical companies and research institutions find and enroll study participants through online platforms, AI-powered matching systems, and data-driven outreach strategies.
Key components include:
- Social media advertising – Targeted Facebook, Instagram, and Google ads reaching specific patient populations
- AI-powered patient matching – Machine learning algorithms that screen electronic health records and patient databases
- Digital consent platforms – Electronic consent systems that streamline enrollment
- Patient registries and databases – Opt-in platforms connecting motivated volunteers with relevant studies
- Remote monitoring tools – Wearables, mobile apps, and telemedicine enabling decentralized trial participation
More than 80% of clinical trials fail to meet their scheduling targets, largely due to recruitment challenges. Failed trials can cost between $800 million to $1.4 billion. Traditional methods like physician referrals and print advertising can’t keep pace with today’s complex study requirements.
Digital recruitment strategies are proving their worth. Facebook ads can boost enrollment rates from 0.8% to 9.4% in large remote trials. Machine learning screening algorithms have cut patient prescreening time from 4 to 2 hours while remaining cost-neutral after enrolling just 12 patients.
As CEO and Co-founder of Lifebit, I’ve spent over 15 years working at the intersection of AI, genomics, and healthcare data platforms – developing solutions that enable more efficient digital clinical trial recruitment through federated data analysis and secure patient matching systems.
Why Traditional Recruitment Falls Short
Traditional recruitment methods – physician referrals, newspaper ads, and site-based outreach – aren’t meeting today’s demands. 86% of clinical trials fail to meet their enrollment goals, and 32% of Phase III trials fail specifically because they can’t find enough participants.
Cancer research shows the severity: less than 5% of cancer patients actually enroll in clinical trials despite potential benefits. This statistic becomes even more concerning when considering that oncology trials often represent patients’ best hope for accessing cutting-edge treatments.
Traditional methods are like filling a swimming pool with a garden hose. Physician networks can only reach patients during scheduled appointments. Print advertising faces declining readership – newspaper circulation has dropped by over 50% in the past decade. Site-based recruitment depends on people walking through the door or being in existing databases.
The geographic limitations are particularly stark. A recent analysis of cancer centers showed that 75% of potential participants live more than 50 miles from the nearest research site. For rare disease studies, this distance often extends to hundreds of miles, creating impossible barriers for many patients.
Hidden Costs of Conventional Methods
When trials can’t meet enrollment targets, sponsors face extended study timelines, additional site fees, protocol amendments, and potentially missed market windows costing hundreds of millions in lost revenue. Each month of delay in bringing a blockbuster drug to market can cost pharmaceutical companies up to $8 million in lost sales.
Site staff time becomes expensive. Manual screening processes consume enormous personnel resources. A typical Phase III trial coordinator spends 60-70% of their time on recruitment activities, from reviewing charts to conducting phone screenings. Staff spend hours reviewing charts and conducting preliminary assessments, driving up operational costs while limiting how many potential participants can be evaluated.
The administrative burden compounds these costs. Paper-based consent processes require multiple in-person visits, extensive filing systems, and manual data entry. Document management alone can consume 20-30% of site coordinator time, reducing their availability for actual patient interaction.
The travel burden on participants creates significant dropout before enrollment. Studies show that 40% of interested patients never complete screening due to travel requirements. Geographic limitations severely restrict the available patient pool, especially for rare disease studies where the total eligible population might be scattered across continents.
Barriers to Diversity & Inclusion
Traditional recruitment has a diversity problem that goes beyond simple demographics. Physician networks and site-based recruitment often reflect existing healthcare disparities, unintentionally excluding patients without regular access to specialized medical care. Academic medical centers, where most trials occur, typically serve more affluent, insured populations.
Socioeconomic factors create multiple barriers. Hourly workers can’t afford time off for multiple screening visits. Patients without reliable transportation face impossible logistics. Childcare responsibilities prevent many potential participants from even considering enrollment.
Language barriers compound this challenge. Most materials are English-only, immediately excluding large portions of potential participants. Even when translations exist, cultural nuances around medical decision-making, family involvement, and trust in healthcare systems require more sophisticated approaches than simple language conversion.
Cultural factors and historical wariness of medical research require more thoughtful, culturally sensitive approaches. The legacy of unethical research practices, from Tuskegee to more recent controversies, has created justified skepticism in many communities that traditional recruitment methods fail to address.
The digital divide presents both challenges and opportunities. While some populations have limited internet access, smartphone adoption has exploded across demographics. Six in ten Americans search for health information online annually – a massive opportunity for inclusive recruitment strategies. Mobile-first approaches can reach populations that traditional methods miss entirely, particularly younger demographics and communities with limited access to traditional healthcare systems.
Core Technologies Powering Digital Clinical Trial Recruitment
Digital clinical trial recruitment has transformed from a slow, paper-heavy process into sophisticated AI-powered systems. Scientific research on digital technologies shows AI-powered systems now identify potential study participants in minutes rather than months.
Federated data platforms solve healthcare’s biggest puzzle: analyzing data from multiple sources without compromising patient privacy. Our Lifebit platform’s Trusted Research Environment (TRE) and Trusted Data Lakehouse (TDL) enable researchers to access global biomedical data while keeping everything secure. These platforms can simultaneously analyze data from dozens of healthcare systems, identifying patterns and potential participants across vast networks without ever moving sensitive information from its secure location.
Electronic health record mining uses machine learning algorithms to scan thousands of patient records, identifying perfect study candidates based on medical history, lab results, and treatment patterns. Natural language processing can interpret physician notes, extracting relevant clinical information that structured data fields might miss. Advanced algorithms can identify subtle patterns – like medication adherence rates or disease progression markers – that human reviewers might overlook.
Patient portals and mobile applications allow participants to complete consent forms on phones, answer questionnaires from home, and stay connected through automated messaging. Modern platforms support multimedia consent processes, including video explanations and interactive elements that improve comprehension compared to traditional paper forms.
Blockchain technology enables patient-to-trial matching in under two seconds while creating unchangeable records of every recruitment step. Smart contracts can automatically execute matching algorithms while maintaining complete audit trails, essential for regulatory compliance and data integrity.
Cloud-based infrastructure provides the scalability needed for global recruitment campaigns. Modern platforms can handle sudden spikes in interest – like those following news coverage of breakthrough treatments – without performance degradation.

Social Media Engines
Facebook reaches over 3 billion people through sophisticated targeting based on location, age, interests, and life events. The platform’s health-specific targeting options include people who’ve shown interest in specific conditions, follow relevant patient advocacy groups, or have engaged with health-related content. Lookalike audience features find people sharing characteristics with current participants, expanding potential pools for rare disease studies.
Instagram’s visual storytelling builds trust through facility tours, patient testimonials, and behind-the-scenes content, particularly effective for younger demographics. Video content performs exceptionally well, with research facility tours generating 3x higher engagement than static images.
Google Paid Search reaches patients when they’re actively searching for treatment information, appearing when someone types “new treatments for diabetes.” Search intent data provides powerful insights into patient needs and concerns, enabling more targeted messaging.
LinkedIn proves valuable for reaching healthcare professionals who might refer patients, while TikTok increasingly reaches younger patient populations through health influencer partnerships.
Patient Databases & Registries
Opt-in patient databases contain people who’ve already expressed interest in clinical research. These platforms often include detailed health profiles, medication histories, and specific research interests. Real-time matching systems work like sophisticated dating apps, comparing inclusion criteria against participant profiles and sending targeted invitations within minutes.
Disease-specific registries provide access to highly motivated patient populations. The Michael J. Fox Foundation’s Fox Trial Finder has connected over 150,000 Parkinson’s patients with research opportunities. Similar registries exist for conditions from diabetes to rare genetic disorders.
Patient advocacy groups provide access to highly engaged communities, particularly effective for rare disease recruitment where traditional advertising might not reach enough potential participants. These partnerships require authentic relationship-building and genuine commitment to community benefit.
AI, Machine Learning & Automation
Natural language processing algorithms automatically identify potential matches within massive databases. These systems can interpret complex medical terminology, understand context, and identify relevant patients even when information is recorded in different formats across various systems.
Predictive analytics help identify patients most likely to complete studies, reducing dropout rates and improving overall trial efficiency. Machine learning models can analyze historical data to predict which participants will remain engaged throughout lengthy study periods.
AI-powered prescreening has cut patient evaluation time from 4 hours to 2 hours while remaining cost-neutral after enrolling just 12 patients. These systems can handle initial eligibility screening, schedule appointments, and even conduct preliminary assessments through chatbot interfaces.
Our Lifebit Patient Management platform demonstrates how federated AI improves recruitment by analyzing genomic, clinical, and real-world data simultaneously.
Automated communication workflows nurture potential participants through personalized sequences while freeing study coordinators for higher-value activities. These systems can send appointment reminders, answer common questions, and maintain engagement throughout the recruitment process.
Designing High-Performing Digital Recruitment Campaigns
Successful digital clinical trial recruitment campaigns require thoughtful planning and continuous refinement. Audience segmentation forms the foundation – a 70-year-old cardiac patient browses differently than a 25-year-old with a rare genetic condition.
A/B testing becomes essential. Small tweaks to headlines, images, or call-to-action buttons can dramatically improve results. Changing a single word in headlines can boost click-through rates by 40%.
Landing pages need to load in under 3 seconds, look great on mobile, and clearly explain participation. Search engine optimization ensures patients googling their condition can find studies organically.
Email marketing remains effective when done right. Optimal sending times fall between 9 AM and 3 PM, and personalized subject lines can double open rates.

Crafting Compelling Study Messaging
Most clinical trial messaging sounds robotic. Patients don’t connect with “investigate the efficacy of novel therapeutic interventions.” They want to know how this study might help them feel better.
Plain language is essential. Instead of “evaluating pharmacokinetic parameters,” try “seeing how the medication moves through your body.”
Value propositions need authenticity. Generic statements about “advancing medical science” don’t motivate. What motivates is access to promising treatments, comprehensive health monitoring, or helping future patients.
Cultural tailoring goes beyond translation. Different communities have varying attitudes toward medical research and decision-making processes.
Video content builds trust through virtual tours of facilities, coordinator interviews, and testimonials from previous participants.
Compliance Checklist & IRB Approvals
Every piece of patient-facing content needs IRB approval before going live, including social media posts.
HIPAA compliance requires robust encryption, strict access controls, and detailed audit logs. Our secure data platforms handle these requirements automatically.
GDPR requirements apply to any European recruitment, requiring explicit consent for data processing and clear privacy notices.
Social media platform policies add complexity. Facebook and Google have specific health advertising rules, including advertiser verification requirements.
eConsent platforms must meet regulatory standards while providing additional protections like timestamping and secure storage.
Ensuring Diversity, Compliance & Trust
Building trust with diverse patient populations is essential for meaningful research. The FDA’s diversity action plans guidance requires intentional strategies that actively engage underrepresented communities. Recent regulatory updates emphasize that diversity isn’t just ethically important – it’s scientifically necessary for developing treatments that work across all populations.
Digital clinical trial recruitment opens doors traditional methods keep closed by meeting patients where they already spend time online. However, this requires understanding that different communities have different digital behaviors, preferred platforms, and communication styles.
Different communities have different relationships with healthcare and medical research. Some populations have valid reasons for caution given historical abuses like the Tuskegee experiments, forced sterilizations, and other unethical research practices. Digital platforms provide new ways to build bridges and demonstrate that modern research prioritizes participant safety through transparent communication and community engagement.
Remote participation options are game-changers for underserved populations. When someone can participate through telemedicine instead of traveling hundreds of miles, we remove barriers unrelated to their willingness to help advance science. This is particularly important for rural communities, where the nearest research site might be in another state.
Economic barriers often prevent participation in traditional trials. Digital approaches can reduce costs for participants by eliminating travel expenses, reducing time off work, and providing flexible scheduling options that accommodate various work schedules and family responsibilities.
Strategies to Reach Underrepresented Populations
Geo-fenced advertising focuses efforts on areas where underrepresented populations live, working best when combined with partnerships with trusted local organizations. Advanced targeting can identify specific zip codes, census tracts, or even individual neighborhoods with high concentrations of target demographics.
Community health worker partnerships provide trusted intermediaries who understand local concerns and can address questions in culturally appropriate ways. These partnerships require long-term relationship building and genuine commitment to community benefit beyond individual studies.
Influencer partnerships provide credible endorsements from trusted community leaders, patient advocates, or healthcare providers with genuine community connections. Micro-influencers often prove more effective than celebrity endorsements, particularly when they have authentic connections to specific health conditions or communities.
Culturally targeted video content addresses specific concerns within different communities. For Hispanic populations, this might emphasize family involvement in healthcare decisions and include Spanish-language materials developed with community input. For African American communities, it often means acknowledging historical medical research abuses and explaining current safety protections, oversight mechanisms, and participant rights.
Faith-based organization partnerships can provide access to trusted community networks, particularly important in communities where religious leaders play significant roles in health-related decision-making.
Telehealth onboarding eliminates transportation barriers preventing rural patients from participating. Mobile health units and pop-up clinics can bring research opportunities directly to underserved communities.
Flexible scheduling options accommodate various work schedules, including evening and weekend availability for people who can’t take time off during traditional business hours.
Data Privacy & Security Foundations
Encryption of data both in transit and at rest provides fundamental security, while zero-trust access controls ensure only authorized personnel access participant information. Modern encryption standards make intercepted data essentially impossible to decode without proper authorization.
Multi-factor authentication adds additional security layers, requiring multiple forms of verification before accessing sensitive systems. Biometric authentication, hardware tokens, and time-based codes provide robust protection against unauthorized access.
Federated governance models enable secure collaboration across institutions while keeping local control over sensitive data. Our Lifebit platform’s federated architecture allows analysis without requiring data to leave its original secure location, addressing privacy concerns while enabling powerful multi-site research.
Regular security audits and penetration testing ensure systems remain secure against evolving threats. Third-party security assessments provide independent verification of protection measures.
Audit logging provides complete transparency about data access, essential for regulatory compliance and identifying potential security breaches. These logs track every interaction with participant data, creating comprehensive accountability trails.
Participant control mechanisms allow individuals to see exactly how their data is being used, update preferences, and withdraw consent at any time. Transparency builds trust and ensures participants maintain control over their personal information.
Measuring Success & Future Trends
Digital clinical trial recruitment transforms success measurement beyond simple enrollment numbers. Cost per enrolled participant often shows higher upfront costs but superior value against actual enrollments.
Conversion funnel analysis tracks every step from initial ad impression to final enrollment, revealing exactly where potential participants drop off.
Real-time analytics enable campaign adjustments within hours rather than waiting weeks for monthly reports.
Retention analytics help predict which participants will complete studies, with machine learning models identifying early disengagement warning signs.
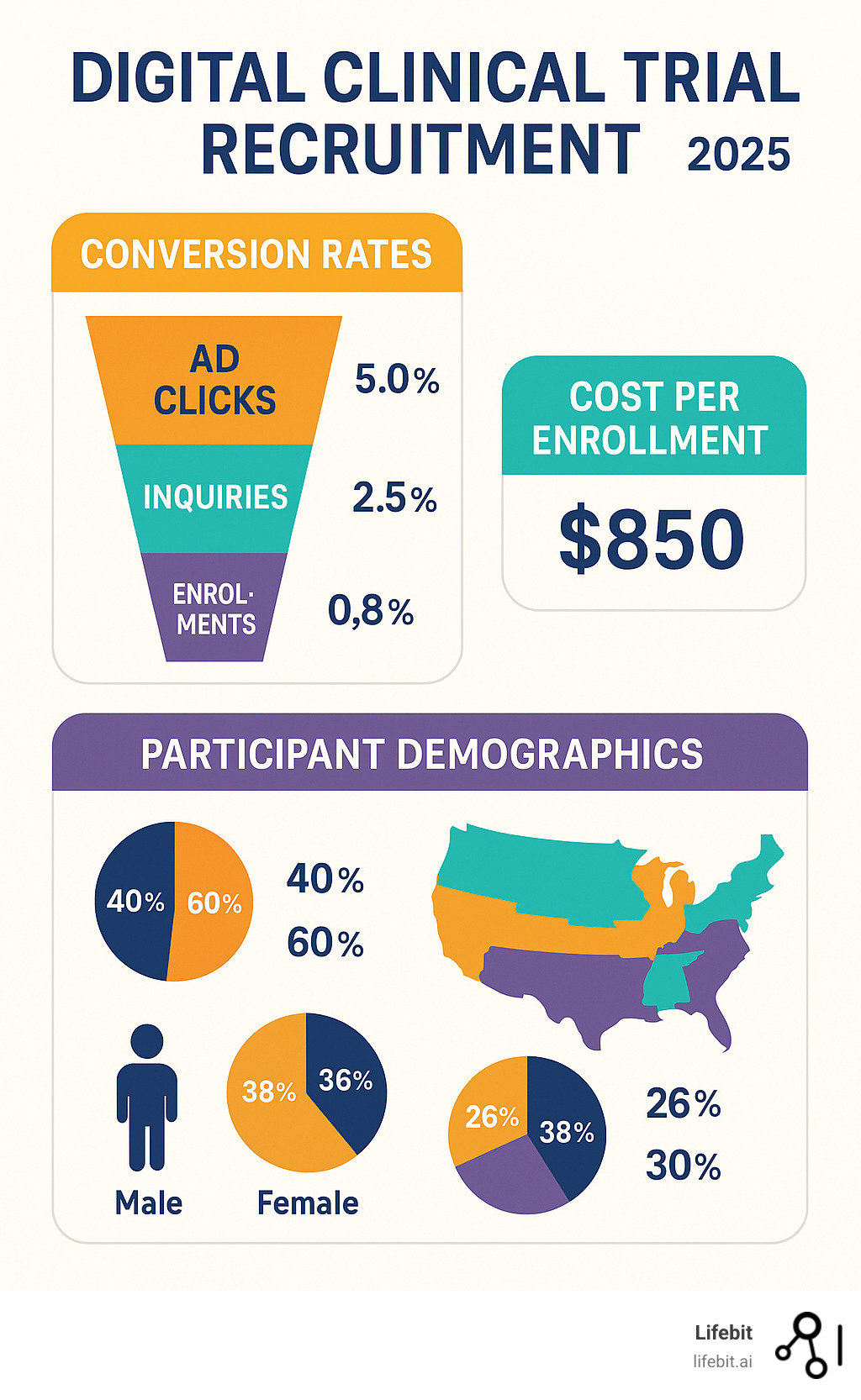
Key Metrics Dashboards
Modern dashboards provide instant visibility into performance. Click-through rates and conversion metrics reveal whether content resonates with audiences. Geographic distribution maps ensure recruitment reaches intended populations.
Demographic tracking becomes crucial for diversity goals, requiring continuous monitoring to address gaps quickly.
Our innovations in clinical trial recruitment and enrollment demonstrate how advanced analytics provide deeper insights into recruitment performance and participant behavior patterns.
What’s Next for Digital Clinical Trial Recruitment
5G networks will enable real-time remote monitoring that seemed impossible years ago, making truly decentralized trials reality.
Conversational AI will provide 24/7 participant support, answering complex eligibility questions while maintaining human touch that builds trust.
Precision targeting represents the next frontier. Combining genomic data, real-world evidence, and predictive analytics will identify ideal candidates with unprecedented accuracy.
Virtual reality consent processes could revolutionize participant understanding through immersive experiences helping people grasp study procedures, risks, and benefits.
Wearable devices and IoT sensors will blur lines between recruitment and participation through continuous health monitoring.
Omnichannel automation will seamlessly coordinate recruitment across multiple platforms while maintaining personalized experiences.
Frequently Asked Questions about Digital Clinical Trial Recruitment
How do digital strategies reduce recruitment timelines?
While traditional methods take weeks to identify potential participants, AI-powered systems scan thousands of patient records in minutes to find matches.
Instead of manual chart reviews and individual phone calls, automated systems send personalized invitations instantly upon identifying eligible participants. Machine learning screening algorithms have cut screening time from 4 hours to 2 hours while maintaining accuracy.
Digital pre-screening questionnaires let participants check eligibility before clinic visits, eliminating screen failures. Parallel processing handles identification, contact, and screening simultaneously across hundreds of potential participants.
What safeguards protect participant data online?
Modern digital clinical trial recruitment platforms use multiple protective layers often exceeding traditional paper-based security.
Encryption protects data both in transit and storage. Access controls ensure only authorized people see participant information based on their specific role.
Our Lifebit platform uses federated data architecture – analysis happens where data already lives securely rather than moving sensitive information around.
Audit trails record every interaction with participant data, showing who accessed what information, when, and why. These digital footprints are more comprehensive than traditional paper records.
Regular security assessments ensure platforms meet strict regulatory requirements including HIPAA, GDPR, and FDA guidelines.
Can digital tools really improve trial diversity?
Digital clinical trial recruitment offers unique ways to reach communities traditional methods often miss entirely.
Geo-targeted advertising focuses specifically on neighborhoods with high concentrations of underrepresented populations. Social media provides access to diverse groups who might never interact with traditional healthcare recruitment channels.
Remote participation options eliminate travel barriers, opening doors for rural communities, people with transportation challenges, and those unable to take time off work.
The key is designing campaigns with genuine cultural sensitivity and community input through multilingual content, culturally targeted messaging, and partnerships with trusted community organizations.
Conclusion
The shift from traditional to digital clinical trial recruitment has become essential for survival in today’s drug development landscape. With over 80% of trials missing enrollment deadlines and failed studies wasting up to $1.4 billion, traditional approaches simply don’t work anymore.
The evidence speaks for itself. Facebook campaigns have transformed enrollment rates from 0.8% to 9.4% in large-scale trials. AI screening tools have slashed prescreening time from 4 hours to 2 hours. These are real results happening now in research centers worldwide.
What excites me most is how these technologies create something more powerful than their individual parts. Instead of juggling separate tools for identification, screening, consent, and communication, we’re moving toward unified platforms handling the entire recruitment journey seamlessly.
Our Lifebit federated AI platform demonstrates this integration. Through our Trusted Research Environment (TRE) and Real-time Evidence & Analytics Layer, researchers can securely access and analyze global biomedical data in real-time, finding the right patients faster while keeping information completely protected.
The future is arriving rapidly. 5G networks will enable unimaginable remote monitoring capabilities. Conversational AI will provide round-the-clock participant support. Virtual reality might help people truly understand study participation before consenting.
Companies and research institutions embracing these digital tools today will have massive advantages as clinical trials become more complex and competitive. Those still relying on newspaper ads and phone calls will find themselves increasingly left behind.
If you’re ready to explore how federated platforms can revolutionize your recruitment efforts, learn more about clinical trial technology trends and find how next-generation biomedical research is happening today.

