The In Silico Revolution: How Computers are Transforming Drug Discovery

What is In Silico Research?
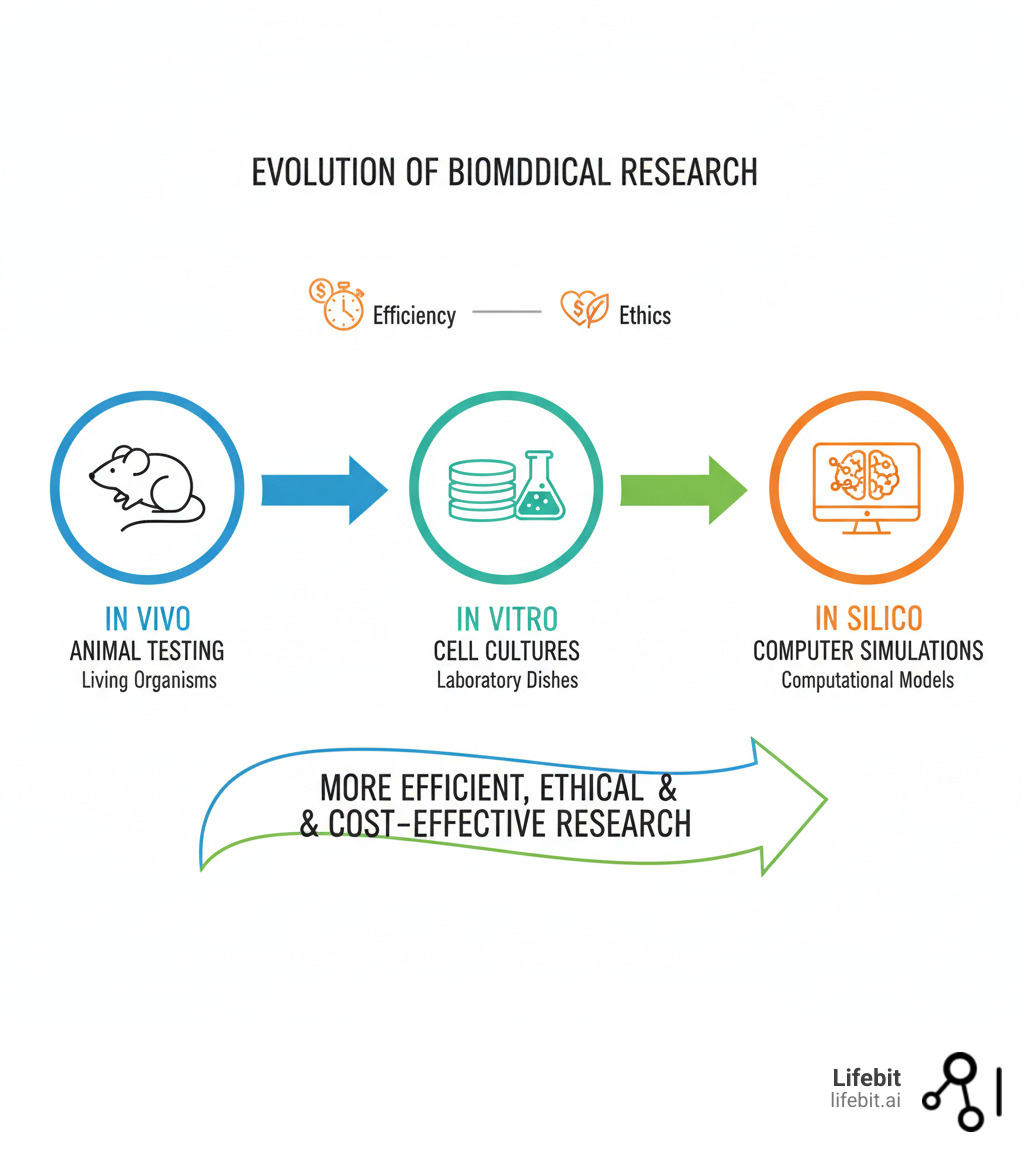
In silico research refers to studies performed entirely through computer simulations and computational models. The term comes from “silicon”—the material in computer chips—and mirrors the familiar scientific phrases “in vivo” (in living organisms) and “in vitro” (in lab dishes).
Quick Definition:
- In silico = Computer-based biological experiments and simulations
- Purpose = Model complex biological processes without live subjects
- Origin = First used by mathematician Pedro Miramontes in 1989
- Applications = Drug findy, disease modeling, protein folding prediction
This computational approach has emerged as the fourth pillar of biomedical research. Instead of testing drugs on animals or cells, scientists can simulate molecular interactions, predict drug toxicity, and model entire disease processes on powerful computers.
The stakes are immense. Traditional drug development can take over a decade and cost billions, with most candidates failing late-stage trials. In silico methods promise to slash these timelines and costs. For example, virtual screening can now analyze 100,000 molecules per day, narrowing massive chemical libraries down to a handful of promising candidates. This approach has already yielded impressive results; in one study, 50% of virtually identified cancer drug candidates proved active when tested in the lab.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’re building the infrastructure to make in silico research more accessible through federated data analysis and AI-powered platforms. With over 15 years in computational biology and genomics, I’ve witnessed how in silico methods are changing everything from drug findy to personalized medicine.
The Rise of Computational Science: From Concept to Core Method
The term “in silico” was coined in 1989 by mathematician Pedro Miramontes, marking the dawn of biology’s digital age. This new field grew alongside the exponential increase in computing power described by Moore’s Law, which predicted that the number of transistors on a microchip would double approximately every two years. This rapid technological advancement transformed computers from simple data storage devices into powerful engines for simulating entire biological systems.
The real catalyst, however, was the explosion of data from large-scale genome sequencing projects, most notably the Human Genome Project (HGP). Completed in 2003, the HGP generated an unprecedented volume of information—over three billion base pairs of human DNA. Traditional lab methods, designed for single-gene or single-protein analysis, were completely overwhelmed. This data deluge created a critical need for new computational approaches to organize, search, and, most importantly, interpret this information. This gave rise to bioinformatics, the interdisciplinary field that develops methods and software tools for understanding biological data. Shortly after, systems biology emerged, using this data to build holistic models that explain how entire biological networks—from metabolic pathways to cellular communication systems—function as a whole, rather than studying genes or proteins in isolation.
The ultimate goal of this field is whole-cell simulation—creating complete, dynamic digital models of living cells that account for every molecule and interaction. A landmark achievement was the 2012 publication of the first whole-cell model of Mycoplasma genitalium, the world’s smallest free-living bacterium. These simulations provide an unparalleled window into the invisible world of molecular biology, allowing us to watch processes unfold in real-time and test hypotheses that would be impossible to explore in a wet lab.
How Computational Models Aid Biological Understanding
In silico models have transformed our ability to study complex biology. They allow researchers to:
- Simulate gene-protein interactions: These molecular conversations can be replayed and adjusted to understand their function in health and disease.
- Model cell phenotypes: Scientists can predict how a cell’s physical and functional characteristics (phenotype) will change based on its genetic makeup (genotype).
- Understand disease dynamics: By simulating a disease’s progression at the molecular level, researchers can identify critical pathways and tipping points for intervention.
- Identify therapeutic targets: Millions of potential drugs can be screened virtually against the 3D structure of a disease-related protein to find the most promising candidates for lab testing.
- Predict drug effects: Models can forecast a drug’s Absorption, Distribution, Metabolism, and Excretion (ADME) profile, as well as potential toxicity (ADMET), long before it reaches a living cell. This scientific research on whole-cell simulation highlights the sophistication of these predictions.
The term “in silico” itself was a clever extension of existing scientific language. It joined “in vivo” (in a living organism) and “in vitro” (in glass) to describe experiments happening inside a computer’s silicon chips. Its formal adoption in the 1990s coincided with the growth of bioinformatics, making computational skills as essential as traditional lab techniques for modern biologists.
The Power of In Silico: Advantages and Current Limitations
The rise of in silico methods is a direct response to the challenges of traditional drug findy, which is notoriously slow, expensive, and ethically complex. However, computational modeling is a powerful tool that comes with its own set of limitations.
The Game-Changing Advantages
- Cost Reduction: By screening thousands of compounds virtually, in silico methods dramatically cut the early-stage research costs of drug findy, which can traditionally exceed $4 billion per approved drug.
- Speed and Efficiency: A virtual screening campaign can analyze 100,000 molecules per day, a task that would take months in a traditional lab. This allows researchers to focus on the most promising compounds from the start.
- Scalability: Computational models can explore a vast number of variables—like dosages, timing, and drug interactions—in a fraction of the time it would take to test them experimentally.
- Ethical Considerations: In silico methods provide a powerful alternative to animal testing, aligning with the global push for more humane research. The FDA’s plan to phase out some mandatory animal testing reflects this shift.
- Early-Stage Failure Detection: Roughly 90% of drug candidates fail during development. Virtual models can predict toxicity or lack of efficacy early, allowing researchers to “fail fast” and pivot to better candidates.
The Current Reality Check
Despite these benefits, in silico modeling is not a perfect solution. Key challenges include:
- Data Complexity: Biological systems are incredibly intricate, and capturing all their interacting parts in a model is difficult.
- Model Accuracy: Models are built on simplifying assumptions, meaning they are useful for generating hypotheses but are not perfect predictors of real-world outcomes.
- Validation Requirements: Every in silico result must be rigorously validated against experimental data before it can be trusted for clinical or regulatory decisions.
- Computational Power: High-fidelity simulations demand significant computing resources, which can be a barrier for some researchers.
Comparing Research Methods: In Vivo, In Vitro, and In Silico
This table shows how in silico methods compare to traditional approaches:
| Feature | In Vivo (Living Organisms) | In Vitro (Lab Dish) | In Silico (Computer) |
|---|---|---|---|
| Cost | Very High (animal care, clinical trials) | Moderate (reagents, cell cultures) | Low to Moderate (software, computing power) |
| Speed | Very Slow (long-term studies, trial phases) | Moderate (cell growth, experimental setups) | Very Fast (simulations in minutes/hours) |
| Complexity | Full biological complexity | Reduced complexity (isolated systems) | Variable (depends on model sophistication) |
| Ethical Concerns | High (animal welfare, patient safety) | Low (ethical cell/tissue handling) | Very Low (no direct harm to living organisms) |
| Typical Use Cases | Drug efficacy, clinical outcomes, disease progression | Molecular mechanisms, cell responses, basic assays | Drug screening, target identification, toxicity prediction |
Overcoming the Problems of Computational Modeling
These challenges are being actively addressed. The need for high-quality data is being met by better standardization and secure data platforms. At Lifebit, our federated approach allows researchers to analyze data from multiple sources while maintaining privacy. Model validation is becoming more systematic, and the computational power requirements are being met by scalable cloud infrastructure. Finally, growing regulatory acceptance, such as the FDA’s plan to phase out some animal testing, signals a clear path toward wider adoption.
Key In Silico Techniques Revolutionizing Drug Findy
The drug findy pipeline is a long, costly, and high-risk journey. In silico methods are revolutionizing this process at nearly every stage, from identifying novel biological targets to optimizing lead compounds. Instead of blindly testing millions of compounds in the lab, scientists use computer models to predict which molecules are most likely to succeed, dramatically improving the odds and focusing resources where they matter most.
Common in silico methods in pharmacology
A powerful toolkit of computational techniques is changing how we develop new medicines:
- Virtual Screening (VS): A foundational technique where algorithms rapidly screen enormous digital libraries of compounds against a biological target. It includes structure-based virtual screening (SBVS), which uses the 3D structure of the target, and ligand-based virtual screening (LBVS), which uses the properties of known active molecules. This process can assess up to 100,000 molecules per day.
- Molecular Docking: The engine behind SBVS, molecular docking simulates how a ligand fits into a protein’s binding site. It generates multiple poses (orientations) of the ligand and uses a ‘scoring function’ to estimate the binding affinity, allowing millions of compounds to be ranked by their predicted potency. Tools like AutoDock and Glide are industry standards.
- Molecular Dynamics (MD) Simulations: While docking is static, MD simulations provide a dynamic view. By applying the laws of physics, these simulations model the movement of every atom in the protein-ligand complex over time. This reveals crucial information about binding stability and protein flexibility, offering a deeper understanding of the molecular interaction.
- Quantitative Structure-Activity Relationship (QSAR): When a target structure is unknown, QSAR models build a mathematical correlation between the chemical structure of molecules and their biological activity. Using machine learning, the model ‘learns’ which molecular features are important for activity from a known dataset, then predicts the activity of new compounds before they are synthesized.
- Pharmacophore Modeling: This technique identifies the essential 3D arrangement of functional groups (e.g., hydrogen bond donors, charged groups) required for a molecule to interact with its target. This ‘pharmacophore’ serves as a 3D search query to find matching molecules in a database or as a blueprint for designing new ones.
- ADMET Prediction: A great drug must be more than just potent; it needs favorable drug-like properties. ADMET models computationally forecast a compound’s Absorption, Distribution, Metabolism, Excretion, and Toxicity. Answering these questions early helps filter out compounds that would otherwise fail in expensive preclinical or clinical studies.
A rich ecosystem of software supports these methods, from open-source academic tools to comprehensive commercial platforms. These tools integrate with vast public databases like DrugBank, ChEMBL, and PubChem, providing the high-quality data foundation that makes accurate predictions possible.
The Impact of Virtual Screening on Efficiency
The efficiency gains from virtual screening are revolutionary. Traditional high-throughput screening (HTS) often has a ‘hit’ rate below 1%. In silico virtual screening flips this equation. By computationally screening millions of compounds, researchers can narrow the field to a few hundred promising candidates for experimental testing—a 99% reduction in lab work. The results speak for themselves: in a 2010 study using the protein docking algorithm EADock, 50% of the molecules predicted to be active in silico were confirmed as active inhibitors in the lab. This hit rate is orders of magnitude higher than HTS, dramatically accelerating drug findy timelines and lowering costs.
At Lifebit, we’re taking this efficiency even further through our federated AI platform, which enables secure access to global biomedical data for training more accurate in silico models.
Real-World Impact: Breakthroughs Driven by In Silico Research
Digital simulations are no longer theoretical exercises; they are driving real medical breakthroughs. In silico research is now at the forefront of advances in drug repurposing, personalized medicine, and disease modeling, delivering tangible results that impact patient care.
- Drug repurposing uses computational methods to find new therapeutic uses for existing, approved drugs. By screening known drugs against new targets, this approach can shave years and millions of dollars off development timelines because the safety profiles of these drugs are already established.
- Personalized medicine is being transformed by models that simulate how different treatments will work for individual patients based on their unique genetic and molecular data, moving away from a one-size-fits-all approach.
- Disease modeling allows researchers to simulate everything from the spread of a virus in a population to the formation of protein tangles in Alzheimer’s disease, enabling new ways to test theories and interventions virtually.
Case Study: Advancing Cancer Immunotherapy
Cancer research, with its immense complexity, has greatly benefited from the in silico revolution. Beyond the impressive 50% hit rate of the EADock algorithm in identifying enzyme inhibitors, computational methods are central to rational drug design. A prime example is the development of inhibitors for the IDO1 enzyme, a key target in cancer immunotherapy. Many tumors use the IDO1 pathway to suppress the immune system, creating a protective shield that allows them to grow unchecked. By using molecular docking and other modeling techniques to study the 3D structure of the IDO1 active site, scientists have designed specific molecules that block this enzyme. This inhibition effectively removes the tumor’s shield, allowing the patient’s own T-cells to recognize and attack the cancer. This approach of designing drugs based on structural knowledge is a cornerstone of modern oncology research. You can learn more about this breakthrough in this article about a new computational tool for cancer treatment.
Case Study: Treating Drug-Resistant Tuberculosis
Tuberculosis remains a serious global health threat, made worse by the rise of multi-drug resistant (MDR-TB) and extensively drug-resistant (XDR-TB) strains that are difficult and costly to treat. In silico research has provided new hope on multiple fronts. In a major repositioning breakthrough, researchers used computational analysis to screen a library of approved drugs, leading to the discovery that Comtan, a drug for Parkinson’s disease, could also fight multi-drug resistant tuberculosis. Because Comtan was already known to be safe, this findy could be moved toward patient application much more quickly. You can explore the details in this research on repositioning Comtan for Tuberculosis. On an even more ambitious scale, the creation of an “in silico cell for TB drug findy” in 2007 represented a leap forward. This complete computational model of the bacterium’s metabolic network allowed scientists to simulate how the cell would react to the loss of different genes or the inhibition of different enzymes, helping to identify the most vulnerable points in its biology and dramatically accelerating the search for new drug targets against resistant strains.
Case Study: Accelerating the Response to COVID-19
The COVID-19 pandemic showcased the power and speed of in silico methods on a global scale. Within weeks of the SARS-CoV-2 genome being sequenced, researchers worldwide were using computational tools to:
- Model the 3D structure of key viral proteins like the spike protein and main protease.
- Perform massive virtual screens of existing drug libraries to identify candidates for repurposing. This work helped prioritize drugs like Remdesivir for clinical trials.
- Design novel vaccine candidates by simulating which parts of the spike protein would elicit the strongest immune response. The rapid development of mRNA and viral vector vaccines was heavily supported by this initial computational work.
- Model viral spread and the impact of public health interventions, providing critical data for government decision-making.
This rapid, global, and computationally-driven response was unprecedented and saved countless lives.
The Future is Computational: AI, Digital Twins, and Ethical Frontiers
The future of science is computational, with Artificial Intelligence (AI) and Machine Learning (ML) reshaping how we approach biological questions. This convergence is giving rise to powerful new concepts like digital twins and in silico clinical trials, moving us from generalized medicine to a truly personalized era.
The concept of digital twins exemplifies this future. A digital twin is a dynamic, personalized simulation of an individual, created by integrating their unique multi-omics data (genomics, proteomics, etc.), electronic health records (EHRs), and lifestyle data. These virtual counterparts will allow doctors to test different drugs and dosages on a patient’s digital model to predict efficacy and adverse effects before a single physical dose is administered, making personalized medicine a tangible reality. Projects like the HumMod simulation and the Oncosimulator are pioneering these advanced decision support tools.
This technology enables personalized treatment simulation, helping to answer why a treatment works for some patients but not others. By analyzing these massive datasets, AI can spot complex patterns invisible to humans, leading to more effective and individualized therapies.
At Lifebit, we’re building the infrastructure for this future. Our platform integrates systems biology with multi-omics data analysis, allowing researchers to understand health as a complex, interconnected network. You can explore our Trusted Research Environment for secure data analysis to see how we’re enabling this cutting-edge research.
The future of in silico clinical trials
What if we could test new drugs on virtual patient models before human trials? This is the promise of in silico clinical trials (ISCTs). Regulatory bodies like the U.S. FDA are actively exploring this frontier through initiatives like the Model-Informed Drug Development (MIDD) program, which uses modeling to improve drug development efficiency.
While not a full replacement for human trials, we are entering an era of “Phase In Silico.” In this paradigm, preclinical studies are conducted on virtual patient populations—diverse cohorts of digital twins representing different ages, ethnicities, and genetic backgrounds. This allows researchers to test a drug’s efficacy and safety across a wide demographic spectrum and optimize trial design before recruiting human participants. The Oncosimulator project is a key example, creating platforms to predict treatment outcomes across these virtual patient groups, making subsequent human trials smarter, smaller, and more targeted.
This shift brings significant regulatory challenges in validating and standardizing these virtual trials. Establishing trust and defining the evidence standards for these models is key to unlocking their potential to make drug development faster, cheaper, and safer.
Ethical Considerations and Challenges
As in silico research grows, we must navigate key ethical questions:
- Data Privacy and Security: Building accurate models requires sensitive health data. At Lifebit, our federated governance model addresses this by allowing analysis on data without moving it from its secure source.
- Algorithmic Bias: AI models trained on unrepresentative data can perpetuate health disparities. If a model is trained primarily on data from one ethnic group, it may perform poorly for others, worsening health inequities. Ensuring diverse datasets and fair algorithms is critical.
- Over-reliance on Models: Simulations are powerful but are still simplifications of reality. They must remain tools to guide, not replace, human judgment and experimental validation.
- The “Black Box” Problem: Some AI systems provide answers without explaining their reasoning. For medical applications, transparency is crucial. The push for ‘explainable AI’ (XAI) aims to make these models more trustworthy for clinicians and regulators.
The solution is responsible innovation, maintaining human oversight and ensuring transparency and validation in all our methods.
Conclusion: Embracing the Computational Shift in Science
The shift from purely physical experiments to a hybrid approach including in silico methods is reshaping science. These computational tools are not replacements but indispensable partners, guiding lab experiments and making research more efficient. By enabling virtual screening, personalized modeling, and large-scale data analysis, in silico research accelerates findy and improves outcomes.
This synergy between computational and experimental approaches is where the real magic happens. The change of scientific research is accelerating, driven by AI, machine learning, and federated data analysis. We are moving toward a future where Phase In Silico is a routine part of drug development and digital twins enable personalized treatment simulations.
At the heart of this change lies the future of data-driven medicine, where global datasets can be analyzed securely and collaboratively. This is where platforms like ours become crucial, providing the infrastructure to make in silico research accessible, secure, and scalable.
Lifebit’s role in enabling secure, federated AI for large-scale research reflects our commitment to this computational future. We are building the bridges that connect global biomedical data, advanced AI analytics, and the researchers who need them most.
The computational shift in science is here. The question is no longer if these methods will become mainstream, but how quickly we can harness their full potential. As we refine these tools, we are fundamentally changing what’s possible in medicine and drug findy.
To see how this future is unfolding, find how federated data analysis is changing research and find the possibilities that await.

