Lifebit Trusted Research Environment: Future Survival Guide

The Hidden Data Crisis Blocking Your Precision Medicine Progress
To cure diseases, researchers need vast amounts of biomedical data. But most of it is locked away in institutional silos, blocked by privacy regulations, or rendered unusable by incompatible formats. This is the single biggest bottleneck in precision medicine today. The scale of the problem is staggering. A single human genome is 200 gigabytes, and large-scale projects sequence hundreds of thousands of individuals. This, combined with electronic health records (EHRs), medical imaging, and other ‘omic’ data (proteomics, metabolomics), creates a data explosion that traditional IT infrastructures cannot handle. The data is not just big; it’s fragmented and fiercely protected.
By 2026, this problem will be critical. Healthcare systems are generating zettabytes of data, yet over 65% of clinical trials face delays from data integration challenges. These are not simple IT issues. They are fundamental barriers stemming from a mosaic of incompatible data formats (e.g., VCF, BAM, FASTQ for genomics; DICOM for imaging; proprietary EHR vendor formats), varying data quality, and a lack of standardized terminologies. Worse, genomic databases are not diverse, with nearly 95% of participants in genome-wide association studies being of European ancestry. This systemic bias means that polygenic risk scores are less accurate for other populations, and drugs developed based on this data may be less effective or have unforeseen side effects in non-European groups. The cost is measured in delayed drug discovery—where every day of delay can cost a company over $1 million in lost revenue—and missed therapeutic breakthroughs that could save lives.
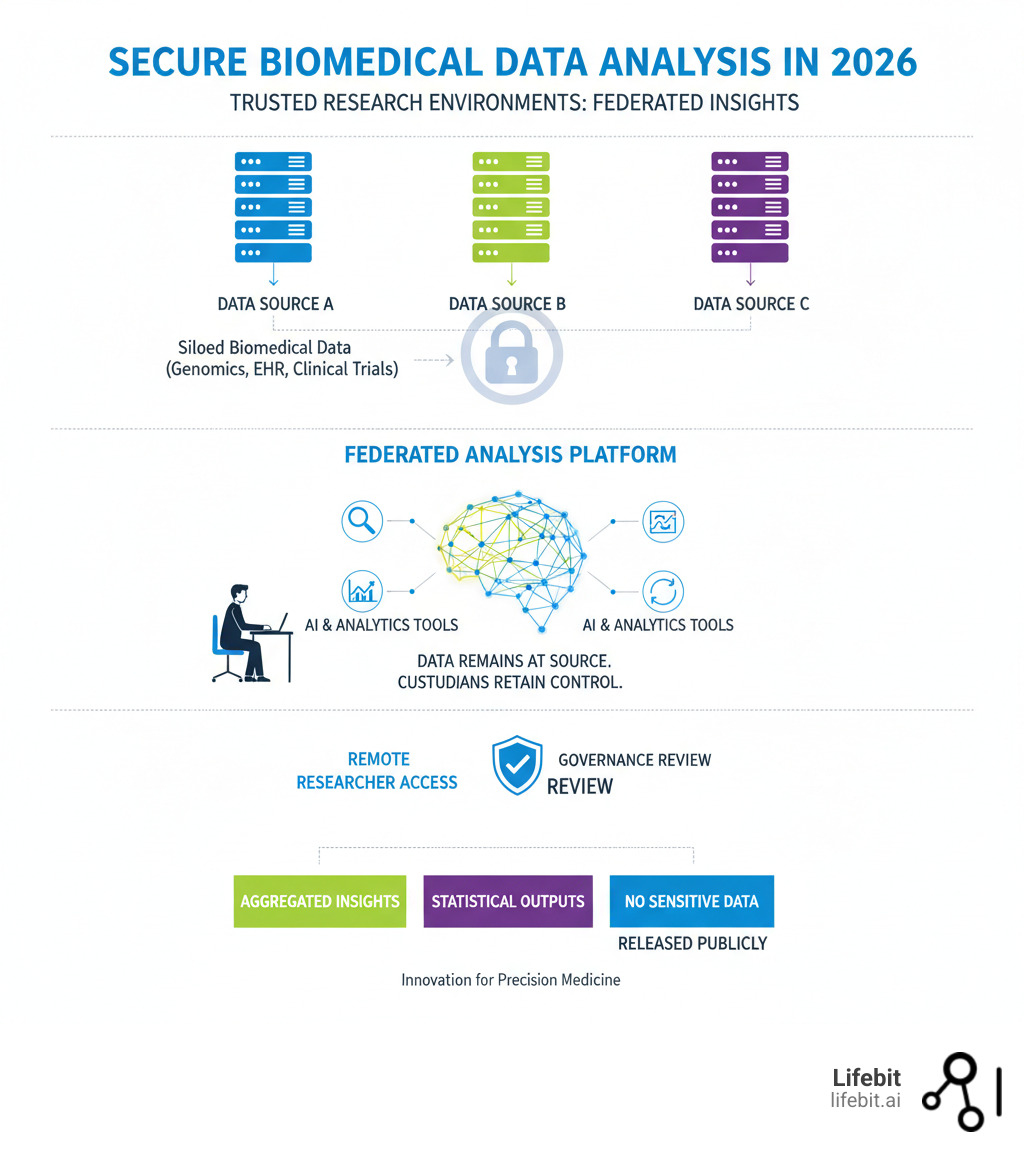
Trusted Research Environments (TREs) are the solution. They provide secure, audited, and collaborative digital spaces where sensitive data can be analyzed without ever leaving its source. The Lifebit Trusted Research Environment in 2026 is a leading federated platform designed for this purpose, enabling researchers to:
- Run federated AI and analytics on distributed data in situ, bringing the analysis to the data.
- Ensure military-grade security with air-locked environments and built-in GDPR/HIPAA compliance.
- Integrate and harmonize multi-omic data (genomics, EHR, imaging, etc.) into analysis-ready formats.
- Collaborate in real-time to unlock insights from previously inaccessible global datasets.
“We’ve spent over a decade building federated platforms to connect pharma to locked-up data,” says Maria Chatzou Dunford, CEO of Lifebit. “The old model of centralizing sensitive patient data into a single data lake is broken. It’s slow, insecure, and doesn’t respect data sovereignty. Understanding our TRE means understanding how federated technology is fundamentally and permanently reshaping biomedical research.”
Basic Lifebit Trusted Research Environment in 2026 terms:
- Data governance platform
- Health data analytics
- Big data analytics
Stop Wasting Years: Why You Need a Trusted Research Environment Now—Or Risk Falling Behind
In 2026, precision medicine research will happen inside secure, compliant environments where data flows freely. Anywhere else, you’ll be stuck in an endless cycle of access requests, data transfer agreements, and institutional roadblocks that can stretch for years. The Lifebit Trusted Research Environment in 2026 was built to end that cycle. It’s a controlled digital workspace where sensitive biomedical data is analyzed without ever leaving its source—bringing the analysis to the data.
At its core, our TRE provides the critical functionalities for cutting-edge research. Instead of spending months negotiating data transfer agreements, researchers can securely access and query genomic data, clinical trial results, and EHRs that would otherwise be inaccessible. Our advanced analysis tools, including GPU-accelerated environments, make complex multi-omics integration and AI/ML modeling fast and efficient. We leverage technologies like NVIDIA Clara Parabricks on AWS to process large-scale genomic data at unprecedented speeds, turning a 30x whole-genome analysis from a 24-hour job into a 25-minute task.

How Lifebit Removes Every Data Roadblock
Every step inside our TRE is controlled, logged, and auditable, transforming the chaotic data access landscape into a streamlined, secure workflow.
- Unified Data Ingestion & Harmonization: Our platform handles the messy reality of biomedical data. It connects to disparate sources and uses a federated ETL (Extract, Transform, Load) process to standardize data against common data models like OMOP and terminologies like SNOMED-CT and LOINC. This transforms raw, multi-modal data (genomics, EHRs, real-world data) into a unified, queryable, and analysis-ready resource without ever centralizing the raw data itself.
- Secure & Portable Analytics: Researchers work in isolated, air-locked sandboxes, which are containerized environments (using technologies like Docker) that are completely sealed off from the public internet. You can bring your existing code, scripts (in R, Python, etc.), and complex Nextflow or WDL pipelines directly into the TRE. The platform ensures your tools can run securely on the remote data, so you can focus on research, not on rewriting code or IT adaptation.
- Granular Governance & Audits: Data custodians retain full, sovereign control. Our platform implements sophisticated role-based (RBAC) and attribute-based (ABAC) access controls, allowing custodians to define precisely who can do what, with which data, and for what purpose. Comprehensive, immutable audit trails log every single action: every login, every query, every script execution, and every parameter used. This provides complete transparency and accountability, building the trust required for collaboration.
- Strict Output Control & Disclosure Prevention: Raw data never leaves the TRE. Only aggregated, non-sensitive results can be exported, and only after passing through a rigorous, custodian-managed approval workflow. The platform has built-in statistical disclosure control mechanisms that check results for any potentially re-identifiable information before they are released. This protects patient privacy to the highest standard while enabling the discovery and sharing of valuable scientific insights.
This federated model makes the data access crisis obsolete. By keeping data distributed and secure, we eliminate transfer risks and simplify compliance with a complex web of global regulations like GDPR and HIPAA. The controlled environment also ensures research is reproducible—a cornerstone of scientific rigor—as every analysis and its environment are versioned and logged. Most importantly, we are breaking down the institutional and political barriers that have fragmented research for decades, enabling collaboration on larger, more diverse datasets that lead to treatments that work for everyone.
Open up Breakthroughs: How Lifebit’s TRE Transforms Research Outcomes by 2026

You know the breakthrough is hiding in the data, but it’s scattered, locked down, and incompatible with your tools. By 2026, this nightmare ends. The Lifebit Trusted Research Environment in 2026 connects you to global biomedical data in real-time, running AI where the data lives to deliver insights that were previously impossible.
Our patented federated technology is the key, allowing pharma companies to analyze patient data across continents without a single byte leaving its secure home. This connects pharma to locked-up data for novel therapeutic insights—the kind that can turn a failed trial into a life-saving treatment. Our TRE integrates advanced AI and machine learning tools directly into the secure environment, letting you unearth complex, non-linear patterns in vast datasets that would take years to find manually.
Open up Insights with Lifebit’s Federated AI
Federated learning is a game-changer for privacy-sensitive fields like genomics. It trains AI models across multiple, decentralized datasets without ever moving or pooling the data. The process is elegant and secure: a global AI model is sent to each data custodian’s secure environment; the model is trained locally on their private data; and only the updated model parameters (anonymous mathematical weights) are sent back to be securely aggregated. This cycle repeats, improving the global model with learnings from all datasets, while the raw data remains protected behind its owner’s firewall. This “data-never-moves” principle is the foundation of our partnership with the Danish National Genome Center, enabling personalized medicine nationwide while keeping patient data under strict Danish control. This approach is more scalable and practical than other privacy-enhancing technologies like fully homomorphic encryption, which remains too computationally expensive for large-scale genomic analyses.
Our platform provides the tools to make this possible:
- AI and ML Analytics: Handle multi-omic data at scale to identify complex genetic architectures, predict disease risk, and find novel therapeutic targets. As noted in scientific research on integrating genomics into healthcare, these capabilities are essential for moving beyond single-gene-disease associations and tackling complex, polygenic conditions.
- Automated Data Harmonization: We automatically standardize disparate datasets, translating different formats and terminologies into a single, analysis-ready resource. Using ontologies and common data models, we create semantic interoperability, meaning a query for ‘myocardial infarction’ can retrieve the correct data from systems that code it differently, drastically reducing manual data cleaning efforts.
- Real-World Evidence (RWE): Securely analyze real-world data (from EHRs, claims, and patient registries) across federated hospital networks. This allows for invaluable insights for pharmacovigilance, comparative effectiveness research, and drug safety monitoring that you can’t get from the controlled, artificial environment of clinical trials alone.
Powering National Precision Medicine with Lifebit
National precision medicine initiatives, like the UK’s Genomics England and the US All of Us program, aim to integrate genomic insights into routine healthcare for entire populations. The Lifebit TRE is designed to be their backbone. These programs face immense challenges: upholding data sovereignty laws, earning and maintaining public trust, building scalable and sustainable infrastructure, and ensuring equitable access and benefit for all citizens. A federated TRE is the only architecture that can solve these challenges simultaneously.
Our federated TRE opens up national health datasets that sit unused due to privacy concerns, enabling researchers to generate novel therapeutic insights. This directly supports ambitious efforts like the European “1+ Million Genomes” Initiative, which requires cross-border data analysis while respecting each nation’s laws. Our partnership with the Danish National Genome Center is a prime example, delivering personalized medicine nationwide by establishing a nationally coordinated, federated infrastructure on our platform. Similarly, we enable research on data from Qatari and Arab populations, helping to close the diversity gap in genomics. By 2026, the countries using our platform won’t just be participating in precision medicine—they’ll be leading it.
The Lifebit Advantage: Faster Research, Lower Costs, and Best Security by 2026

By 2026, the Lifebit Trusted Research Environment in 2026 will be the essential platform separating leaders from laggards in biomedical research. Your competitors are already making decisions faster and collaborating more effectively. The question isn’t whether you need a TRE; it’s whether you can afford to use anything less than the best.
Our platform delivers tangible, quantifiable results:
- Faster Research: Cut research cycles from months and years to days and hours. A federated Genome-Wide Association Study (GWAS) across three biobanks, which would typically take 18 months of contract negotiations and data transfers, can be completed in a week. One partner saved three months on a single clinical trial recruitment phase by using our platform to identify eligible patient cohorts across multiple hospitals in real-time.
- Cost Reduction: Our cloud-native, federated architecture eliminates the immense costs of creating and maintaining redundant data copies and the massive data transfer and storage fees associated with centralized data lakes. Organizations report operational savings of 40% or more by avoiding the need for dedicated IT infrastructure and specialized staff for data management.
- Improved Security: Our “data-never-moves” approach is fundamentally more secure. Data stays with its custodian, eliminating the risk of data breaches during transfer. We layer on air-locked environments, granular controls, and continuous audits for security that lets you sleep at night.
- Global Collaboration: We connect researchers across five continents, enabling teams from different organizations, countries, and legal jurisdictions to work together seamlessly within a single secure, compliant environment.
For pharmaceutical companies, this translates into a genuine competitive edge. You can access diverse, real-world datasets, identify and validate drug targets faster, and design more precise and efficient clinical trials. In an industry where being six months ahead can mean billions in revenue, this advantage is everything.
Security and Privacy: A Multi-Layered Defense
The Lifebit TRE treats security as its absolute foundation. Our data protection by design approach, compliant with GDPR Article 25, embeds security into every line of code and architectural decision. This is not a feature; it is the core of the platform.
- Architectural Security: We are engineered for GDPR and HIPAA compliance, meeting the world’s strictest regulations. Our platform undergoes regular penetration testing and holds key certifications like ISO 27001 and SOC 2, Type II, attesting to our rigorous security controls.
- Federated Governance: This is our first line of defense. Control is placed in the hands of the data custodians. They set the rules of engagement, and our platform programmatically enforces them, building the trust necessary for large-scale collaboration.
- Air-Locked Environments: Analyses happen in isolated “clean rooms” or sandboxes. These containerized environments are completely sealed, with no inbound or outbound internet access and disabled clipboard functions, making it architecturally impossible for a user to manually copy or move raw data.
- Granular Access Controls & Audits: We enforce the principle of least privilege. Every interaction is authorized, purposeful, and secure. Immutable audit logs track every query and command, providing a complete, verifiable record of how data was used, which is essential for both security and scientific reproducibility.
- Controlled Data Egress: The only way for information to leave the environment is as aggregated, anonymized results that have been vetted by statistical disclosure controls and explicitly approved by the data custodian. This robust, multi-step process ensures patient privacy is never compromised.
The Collaboration Advantage: Connecting Researchers and Pharma
The biggest breakthroughs happen through collaboration. Our TRE eliminates the historical barriers between academia and industry, providing a neutral ground where both sides can work together without compromising intellectual property or patient privacy.
- Secure Project Spaces: We provide isolated digital hubs where cross-functional teams can share code, analytical pipelines, and discuss findings with complete transparency and audibility, fostering a truly collaborative research ecosystem.
- Shared Analytics Tools: A standardized, validated toolset ensures everyone works from the same playbook. This accelerates research, eliminates variability, and guarantees that results are reproducible across teams and organizations.
- Pre-competitive Research: Our platform is the perfect environment for pharmaceutical consortia to pool resources on foundational science. For example, multiple companies can securely and jointly analyze federated real-world data to validate a new class of biomarkers for patient stratification, accelerating discoveries that benefit the entire industry.
This collaborative model directly accelerates drug discovery and delivers a strong ROI by shrinking time-to-market, reducing clinical trial failure rates, and eliminating duplicated effort and infrastructure costs.
Why Lifebit’s TRE Is Unbeatable for 2026
Many companies promise secure data platforms. Lifebit has delivered at scale for national governments and leading biobanks across continents. Our key differentiators are clear:
- Patented Federated Architecture: This is our core distinction. While other TREs are often just secure virtual machines requiring data to be moved in, our platform eliminates the need to move data at all. This is the only approach that truly scales globally and respects data sovereignty.
- Biobank-Scale Scalability: Our platform is proven to handle millions of whole genome sequences and petabytes of associated data today, powering some ofthe world’s largest national genomics programs.
- Advanced AI/ML Integration: We go beyond basic analytics, integrating sophisticated tools for federated learning that can find patterns invisible to other methods.
- Seamless Interoperability: Using standards like HL7 FHIR and OMOP, our platform seamlessly connects with existing hospital systems, EHRs, and LIMS, ensuring it fits into your ecosystem rather than forcing you to rebuild it.
Your 2026 Roadmap: What You’ll Gain with Lifebit’s Trusted Research Environment
Imagine a 2026 where the data access crisis is solved. This is the reality the Lifebit Trusted Research Environment in 2026 is building today. The digital health landscape is changing fast, with tightening regulations, increasing data volumes, and rising pressure for breakthroughs. Our vision is a world where all sensitive biomedical data can be securely leveraged to cure diseases, no matter where it lives.
By 2026, our TRE will be the standard for precision medicine research, proven by real results. Our technology roadmap is focused on continuous innovation in federated AI, multi-omics integration, and real-time analytics to stay ahead of what researchers need next.
For your organization, this means:
- Accelerated Discovery: Cut research timelines from months to days.
- Broader Data Access: Tap into previously locked, diverse, global datasets.
- AI-Powered Insights: Find novel, complex patterns that human analysis would miss.
- True Global Collaboration: Work seamlessly and securely with international partners.
- Ironclad Security & Compliance: Eliminate risky data transfers and meet GDPR/HIPAA standards by design.
- Significant Cost Efficiency: Optimize computational resources and drastically reduce infrastructure and data transfer spending.
What Researchers Will Achieve by 2026 with Lifebit
Your discovery cycles will accelerate dramatically, moving from question to insight in hours, not weeks. This speed, combined with access to more diverse and representative datasets, leads to more robust, generalizable findings and higher-impact publications in top-tier journals. Your results become bulletproof. With every step of your analysis—from the exact software versions in your containerized environment to the parameters of your script—being transparent, auditable, and logged, your research achieves a new standard of reproducibility. Most importantly, you’ll stop wasting 80% of your time on data wrangling, access requests, and infrastructure headaches, and focus on what you do best: science.
Lifebit’s 2026 Roadmap: What’s Next
Our innovation doesn’t stop. Here’s a glimpse of what’s coming to the platform:
- Improved AI Capabilities: We are developing more sophisticated federated learning models, including graph neural networks to analyze biological pathways and transformer models to interpret the ‘language’ of the genome, enabling the discovery of more complex and novel therapeutic targets.
- Deeper Multi-omics Integration: We are building advanced methods to seamlessly harmonize and integrate genomics, transcriptomics, proteomics, metabolomics, and imaging data. This will provide a truly holistic view of patient biology, allowing researchers to understand the full chain of events from genetic predisposition to disease phenotype.
- Real-time Pharmacovigilance: Our platform will enable continuous, proactive monitoring of drug safety signals. Authorized federated queries will scan real-world data across our network of hospitals for early warnings of adverse events, transforming pharmacovigilance from a reactive to a proactive discipline.
- Expanding Data Network: We are constantly adding more hospitals, biobanks, research institutions, and patient-led data cooperatives to our global network, with a focus on increasing data diversity from underrepresented populations in Asia, Africa, and South America.
- Integrated Clinical Trial Support: We are deepening our capabilities to support the entire clinical trial lifecycle. This includes federated cohort discovery to find and recruit eligible patients in minutes, AI-driven patient stratification to ensure the right patients are in the right trial arms, and using the TRE as the central hub for collecting and analyzing trial data in real-time.
Don’t Get Left Behind—Secure Your Research Future with Lifebit
The clock is ticking. While you read this, your competitors are analyzing data that could lead to the next breakthrough. Are you among them, or are you still waiting for data access approvals and navigating institutional red tape?
The future of precision medicine is unfolding now, and the Lifebit Trusted Research Environment in 2026 is the platform making it possible. Our patented federated technology ensures data custodians keep complete control while researchers gain the insights they need. Analyses happen where data lives—only aggregated, non-identifiable insights leave the environment.
This shift to secure, federated research is no longer optional; it’s essential for survival and leadership. Organizations that adopt this technology today will make the breakthrough discoveries of tomorrow. Federated technology is how national health programs are unlocking their datasets, how pharma companies are accelerating drug discovery, and how researchers are finally accessing the diverse, population-scale data needed to solve humanity’s greatest health challenges.
At Lifebit, we are proud to lead this change, powering national precision medicine initiatives and connecting researchers across five continents. But the data access crisis won’t wait. Every day you delay is a day your competitors get further ahead, and a day a potential discovery remains locked in a silo.
You can continue with the old, broken way—waiting for permissions and centralizing data—or you can join the leading organizations using our TRE to accelerate their research, reduce costs, and secure their future in the new era of medicine.
Discover Lifebit’s federated platform and secure your place in the future of precision medicine. The researchers who win in 2026 will be the ones who can access and analyze the data everyone else can’t reach.


