Medical Trial Software Comparison 101

Why Legacy Trial Software Is a Budget-Draining Nightmare
Medical trial software is revolutionizing how research teams manage clinical studies. The right platform cuts costs and accelerates timelines; the wrong one drains budgets, fragments workflows, and buries teams in manual work.
What modern medical trial software does:
| Core Function | What It Delivers |
|---|---|
| Electronic Data Capture (EDC) | Secure, real-time collection of trial data |
| Site & Patient Management | Enrollment tracking, visit scheduling, participant engagement |
| ePRO/eCOA & eConsent | Patient-reported outcomes and digital consent workflows |
| Decentralized Trial (DCT) Support | Remote monitoring, wearables integration, virtual visits |
| Analytics & Reporting | Real-time dashboards, risk-based monitoring, AI-driven insights |
| Regulatory Compliance | 21 CFR Part 11, GDPR, HIPAA-ready audit trails and version control |
Bringing a new therapy to FDA approval can take over 10 years and cost more than $2 billion. With 93% more drug trials using decentralized or virtual components in 2021, legacy systems simply can’t keep up with the pace or data complexity.
Yet 17% of organizations still have no plans to modernize their clinical trial systems. This is a dangerous gap. Today’s trials demand unified platforms that integrate EDC, ePRO, eConsent, and AI-powered analytics—not fragmented tools that force teams to stitch together data silos.
This guide breaks down what modern medical trial software must deliver, compares the leading platforms, and shows you how to choose the right solution for your trial.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. We’ve spent over a decade building genomics and medical trial software platforms that enable secure, federated analysis of real-world and clinical data. My work spans computational biology, AI, and health-tech entrepreneurship, all focused on making precision medicine faster, safer, and more accessible.
Quick medical trial software terms:
5 Ways Outdated Trial Software Is Sabotaging Your Research
Here’s the uncomfortable truth: most clinical trial failures aren’t about bad science—they’re about bad systems. When data disappears into silos and teams waste hours on manual, low-value tasks, budgets balloon and timelines slip. This isn’t just frustrating; it’s an expensive and entirely preventable drag on medical innovation.
1. Crippling Operational Inefficiency: This is the silent killer of trials. Legacy systems force highly skilled professionals to perform administrative busywork. Imagine a site coordinator spending two hours per day manually transcribing lab results from a PDF into the EDC system. Across 50 sites, that’s 100 hours of wasted effort daily—hours that could be spent on patient care and engagement. Fragmented workflows mean every task, from scheduling a visit to reconciling a payment, costs more, takes longer, and introduces a new opportunity for human error. This administrative burden is a primary driver of site burnout and turnover.
2. Unmanageable Data Management Headaches: Modern trials generate a deluge of data from disparate sources: site-entered EDC data, patient-reported outcomes from apps, lab results from central and local labs, and continuous streams of biometric data from wearables. Without an integrated system, your data management team becomes a human data pipeline, manually exporting, cleaning, and stitching together information in spreadsheets. This process is not only slow and error-prone, but it also creates massive security gaps. When data is exported to an unsecured spreadsheet and emailed, it leaves the secure, audited environment of the platform, creating a potential HIPAA breach and destroying the data’s lineage, making it impossible to trace its origin and transformations during an audit.
3. Poor Patient Recruitment and Retention: Patient recruitment and retention consistently rank among the 8 biggest challenges facing clinical trial professionals. With industry-wide patient dropout rates averaging 30%, keeping participants engaged is paramount. Legacy systems, often designed with only the data manager in mind, provide a clunky, unintuitive patient experience. They lack mobile-friendly interfaces, offer no convenient communication channels, and make participation feel like a chore. High dropout rates are the predictable result, jeopardizing the statistical power of a study and often forcing costly extensions or re-designs.
4. Pervasive Collaboration Breakdowns: Clinical trials are a team sport involving sponsors, CROs, site staff, monitors, and patients. When these stakeholders work from different, disconnected systems, miscommunication is inevitable. A protocol amendment updated in the sponsor’s system may not be immediately visible to a site coordinator, leading to a protocol deviation. A monitor’s query in one tool may go unnoticed by a data manager working in another. These small disconnects compound, leading to delayed decisions, missed critical issues, and stalled progress.
5. Inevitable Regulatory Compliance Failures: Modern regulations like 21 CFR Part 11, GDPR, and HIPAA are not afterthoughts; they demand that compliance be woven into the fabric of a system. This includes immutable audit trails, validated electronic signatures, and granular access controls. Trying to bolt compliance onto an outdated system is like retrofitting seatbelts onto a horse-drawn carriage—it misses the point and is bound to fail. It forces teams to rely on manual SOPs and paper-based logs to fill the gaps, a process that is notoriously unreliable and an auditor’s nightmare.
All of this adds up to skyrocketing costs. Every manual process, data error, and preventable delay drains your budget, which already exceeds $2 billion per approved therapy. Understanding the Current Systems and Technology in Clinical Trials reveals just how wide this gap has become.
Modern medical trial software transforms these pain points into competitive advantages. It acts as your trial’s central nervous system, automating busywork, ensuring data integrity, and giving every stakeholder the right information at the right time. The difference is between reactive problem-solving and predictive analytics that spot issues before they escalate.
Core Capabilities of High-Impact Trial Software
Not all medical trial software is created equal. A high-impact platform is more than a collection of features; it’s an integrated ecosystem designed to accelerate research. It must deliver:
- Electronic Data Capture (EDC): This is the foundation, but it must go beyond simple online forms. A modern EDC provides an intuitive, drag-and-drop interface for building eCRFs, complete with intelligent validation rules (e.g., range checks, logic checks) that prevent data errors at the point of entry. Your EDC in Clinical Research should feel intuitive, not bureaucratic, to maximize site adoption and data quality.
- ePRO and eCOA: Empower patients by putting data collection directly in their hands via their own devices (a BYOD strategy). This delivers richer, more timely, and more accurate patient-reported outcomes and clinical outcome assessments with significantly less site burden. Look for features like customizable surveys, automated reminders, and a user-friendly mobile app.
- eConsent: Transform the consent process from a mountain of paper into a clear, interactive, and trackable digital experience. Modern eConsent uses multimedia elements like videos and comprehension quizzes to ensure patients truly understand the trial. It captures a legally binding electronic signature and creates a complete, time-stamped audit trail for regulatory peace of mind.
- Site and Patient Management: Automate the logistical backbone of your trial. This includes tracking enrollment pipelines, automating visit scheduling, and sending automated reminders to both sites and patients. This frees up clinical research coordinators to focus on high-value patient care and relationship-building instead of administrative tasks.
- Financial Management: Integrate budget tracking, site payments, and patient stipends directly into the platform. This provides real-time visibility into trial financials and eliminates the reconciliation nightmares that plague quarter-end reviews. Automated, milestone-based payments to sites build trust and improve sponsor-site relationships.
- Analytics and Reporting: Move from static, end-of-study reports to dynamic, real-time dashboards. The platform should provide role-based views on enrollment progress, data quality metrics, query resolution times, and site performance. The ability to generate compliant reports in minutes, not days, is a critical efficiency gain.
Why Your Software Must Support Decentralized Trials (DCTs)
The clinical trial world has shifted, and there’s no going back. Decentralized and hybrid trials are the new normal. If your medical trial software can’t support them, you’re already behind.
The numbers tell the story: trials using DCT components grew by 93% in 2021. This shift is about meeting patients where they are, which dramatically expands the potential patient pool, improves retention by reducing travel burden, and collects more ecologically valid real-world data.
But DCTs require a fundamentally different software architecture:
- Remote Data Collection: The system must seamlessly capture ePRO data from patient smartphones and integrate high-volume, high-velocity data streams from consumer and medical-grade wearables (e.g., activity levels, sleep patterns, heart rate, blood glucose).
- Virtual Patient Visits: Secure, HIPAA-compliant video conferencing must be baked into the platform, not offered as a clunky third-party integration. These visits should be automatically logged and linked to the patient’s record.
- Direct-to-Patient Logistics: The software should support workflows for shipping study medication, lab kits, and devices directly to a patient’s home and tracking their return.
Legacy systems built around the assumption of in-person site visits simply can’t handle this distributed architecture. As McKinsey explores in “No place like home? Stepping up the decentralization of clinical trials,” this change demands new operational models and new software. The Decentralized Clinical Trials Software Guide 2025 details the technical requirements. The question isn’t if you’ll adopt DCTs, but if your software is ready.
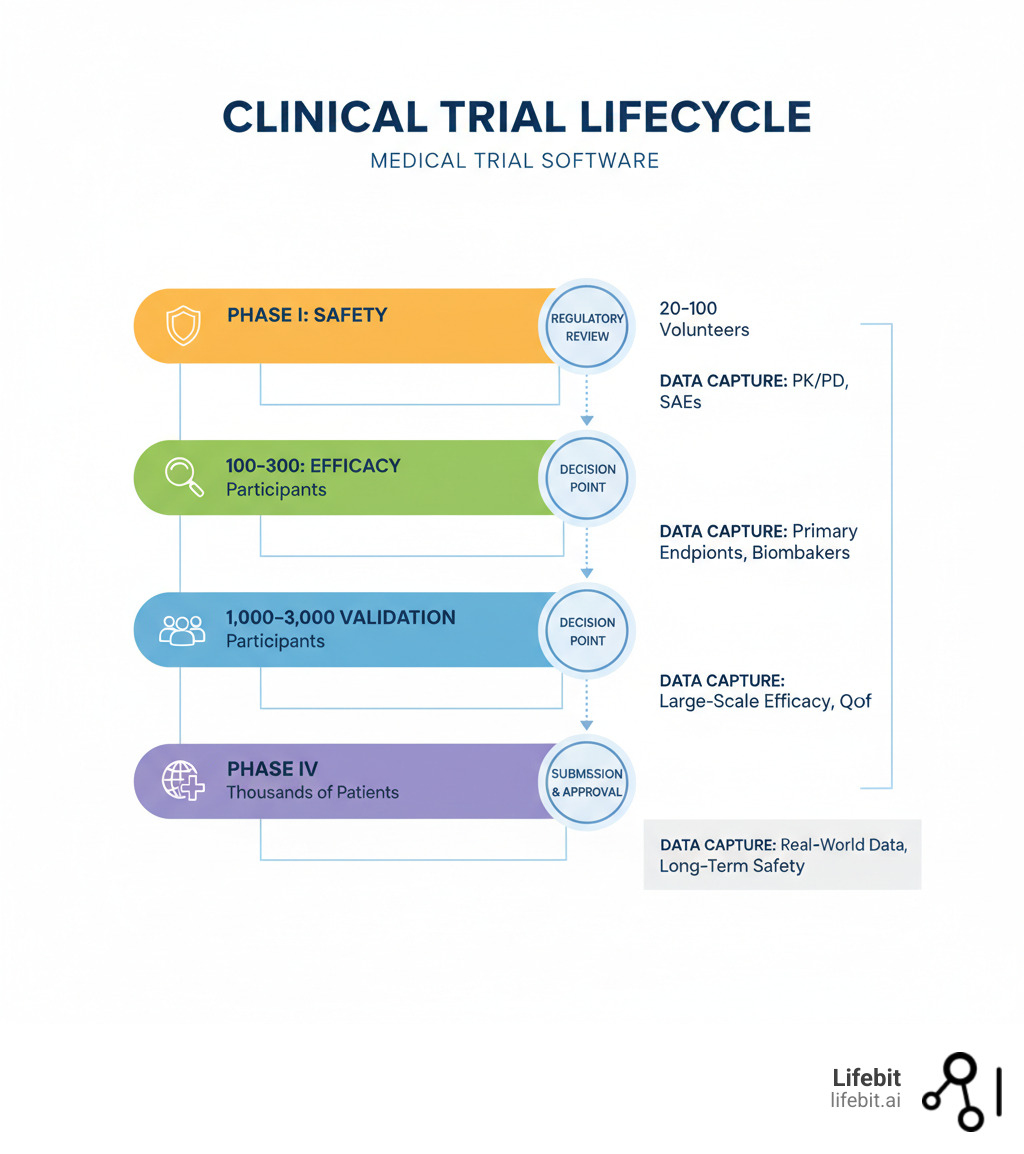
10 Must-Have Capabilities for Your Medical Trial Software in 2025
Choosing medical trial software is a long-term strategic decision that directly impacts your organization’s efficiency, data quality, and regulatory standing. The wrong choice saddles you with technical debt, frustrates your teams, and delays getting vital treatments to patients. This checklist covers the ten non-negotiable capabilities you should demand from any modern vendor.

1. Unified Data Capture and Management
A unified platform is the cornerstone of modern clinical research. This means your Electronic Data Capture (EDC), electronic Patient-Reported Outcomes (ePRO), electronic Consent (eConsent), and Decentralized Clinical Trial (DCT) modules are not just bundled together, but are built on a single, coherent data model. This eliminates the need for complex, brittle integrations between separate systems, ensuring a single source of truth for all trial data.
Look for self-service study build tools that empower your clinical team to design and launch studies quickly using drag-and-drop interfaces and pre-built templates, without requiring constant IT support or custom coding. An intuitive, consistent user interface across all modules is key to driving user adoption, reducing training time, and ultimately, improving data quality.
2. Integrated Site Operations and Financial Management
Your clinical trial sites are your partners, not your vendors. The best software recognizes this by integrating operational and financial management directly into the platform. This goes far beyond simple data capture. Demand automated contract and budget negotiation workflows, streamlined site and patient payments triggered by study milestones, and comprehensive financial reporting.
When sites can manage both their research activities and their business operations in one place, it dramatically reduces their administrative burden and improves the sponsor-site relationship. Transparent, timely payments are one of the most effective ways to become a “sponsor of choice” and ensure your trials are prioritized by high-performing sites.
3. Native Decentralized Trial and AI-Driven Capabilities
Modern platforms are built from the ground up for DCTs and hybrid trials. This means they natively support electronic Clinical Outcome Assessments (eCOA), eConsent, remote monitoring, and telehealth. However, the real competitive advantage comes from the intelligent application of Artificial Intelligence (AI).
AI should be woven into the platform to automate routine tasks, such as sending patient reminders or flagging missing data. More advanced AI can analyze incoming data in real time to spot anomalies, predict patient dropouts, or identify sites that are struggling. For example, an AI algorithm can flag a patient whose self-reported symptom scores are deviating significantly from their baseline, prompting a remote check-in from the clinical team. This delivers a significant ROI by shifting your team’s focus from manual data review to proactive, risk-based management.
4. Intuitive, Fast Study Build and Global Support
Speed is a critical currency in clinical development. The best medical trial software allows you to move from protocol to first-patient-in within weeks, not the six-plus months typical of legacy systems. This is achieved through intuitive, no-code study builders, libraries of reusable forms and templates, and automated validation testing.
High usability ratings (verified by third-party reviews) and a responsive, 24/7 global support team are essential for rapid deployment and smooth operations, especially for global trials spanning multiple time zones. Your software vendor should be a partner in your success, providing expert guidance and support throughout the entire trial lifecycle.
5. Digital Document and Workflow Automation
Eliminate the compliance nightmare of paper binders and manual document tracking. Your software must include an integrated electronic Investigator Site File (eISF) that can seamlessly exchange documents with your electronic Trial Master File (eTMF). This creates a single, authoritative location for all essential documents.
Look for features like secure, 21 CFR Part 11-compliant eSignatures and automated workflows that route documents for review and approval. This builds compliance directly into your process, ensuring you are always audit-ready and dramatically reducing the time and cost associated with monitoring and site closeout.
6. Seamless EDC and EHR Integration
Automated data extraction from Electronic Health Records (EHR) is a game-changer for site efficiency and data quality. Manually transcribing data from a patient’s chart into the EDC (a process known as Source Data Transcription) is a major source of errors and a huge burden on site staff.
Modern platforms tackle this with standards-based integration, primarily using HL7 FHIR (Fast Healthcare Interoperability Resources) APIs. This allows for the automated, near-real-time transfer of structured data (like demographics, labs, and medications) from the EHR directly to the EDC, eliminating redundant data entry. This not only saves time but also significantly reduces the need for costly Source Data Verification (SDV).
7. Enterprise-Grade Scalability and ROI Reporting
Your chosen platform must be able to grow with you, whether you’re a small biotech running your first trial or a large pharma managing a portfolio of hundreds of studies. This requires an enterprise-grade, cloud-native architecture that ensures high availability, robust security (evidenced by certifications like SOC 2 and ISO 27001), and the ability to scale to handle thousands of users and massive data volumes.
Furthermore, the platform must help you prove its value. It needs robust ROI reporting capabilities that quantify the benefits of modernization. This includes dashboards that track metrics like reduced study build time, faster enrollment cycles, lower monitoring costs (e.g., travel savings from remote monitoring), and improved data quality (e.g., fewer queries per patient).
8. Modular, AI-Assisted Data Monitoring
Modern EDC systems are modular and supercharged by AI. This enables a sophisticated Risk-Based Monitoring (RBM) strategy, moving away from the inefficient 100% SDV model. AI-driven insights monitor incoming data in real time, automatically flagging statistical outliers, protocol deviations, and potential fraud.
This allows your clinical monitoring team to focus their attention on the highest-risk areas, rather than manually reviewing every single data point. The system should help you identify Key Risk Indicators (KRIs) and then automate the surveillance of those indicators, allowing your team to manage by exception and intervene before issues become critical.
9. Patient Recruitment and Engagement Automation
Directly tackle the industry’s biggest bottleneck with a suite of patient-centric tools. The software should support the entire patient journey. This starts with automated online screening questionnaires that can be embedded on websites and social media to identify eligible candidates. Once a patient is enrolled, the platform should simplify scheduling and use multi-channel engagement tools (email, text, in-app notifications) to boost retention.
Look for a dedicated patient portal that provides a central hub for participants to complete ePRO diaries, view their visit schedule, access educational content, and communicate with the study team. Keeping patients informed and engaged is the single best strategy for improving adherence and reducing dropout rates. For more proven approaches, check out our guide on Clinical Trial Recruitment Strategies.
10. Integrated Quality and Compliance Management
A robust Quality Management System (QMS) must be an integrated part of the platform, not a separate, siloed application. When a deviation occurs—for example, a temperature excursion for a stored biological sample—the system should allow you to immediately launch a Corrective and Preventive Action (CAPA) workflow.
This integrated approach links the event to the investigation, the root cause analysis, the action plan, and the final verification, with every step captured in the audit trail. The platform must manage documents, CAPAs, audits, and training records while ensuring unwavering adherence to global regulations like 21 CFR Part 11, GCP, GDPR, and HIPAA with secure, unalterable audit trails.
Integrate or Disintegrate: The Future of Trial Software Is AI, Data, and Compliance
The era of disconnected point solutions and manual workarounds is over. The future of medical trial software is built on three pillars: seamless integration, intelligent automation, and unwavering data integrity. As emerging Clinical Trial Technology Trends make clear, unified platforms are no longer a luxury—they are survival tools for any research organization.

That old model of separate systems for scheduling (CTMS), data capture (EDC), and regulatory documents (eTMF)—all held together by a fragile web of spreadsheets and emails—is dying. Good riddance. It was inefficient, insecure, and a barrier to progress.
AI Is Here: How It’s Revolutionizing Clinical Research
Artificial intelligence is no longer a futuristic buzzword; it is actively rewriting the rules of clinical trials today. Our AI for Clinical Trials Complete Guide provides a deep dive, but here’s how it’s making a tangible impact:
- AI-Powered Patient Matching: Traditional recruitment relies on manual chart review, a slow and often incomplete process. AI algorithms can scan thousands of electronic health records in seconds, parsing not just structured data but also unstructured clinician notes and pathology reports to find eligible candidates who would otherwise be missed. We’ve streamlined this with tools like Lifebit Patient Management.
- Predictive Analytics for Risk Mitigation: By analyzing historical and real-time trial data, machine learning models can forecast trial outcomes, predict patient dropouts, and identify sites that are underperforming. For example, a model can flag a patient at high risk of non-adherence based on early patterns of missed ePRO entries, allowing the site team to intervene proactively.
- Intelligent Risk-Based Monitoring (RBM): AI automates the tedious aspects of RBM. It uses statistical algorithms to monitor data streams for anomalies and outliers, focusing your team’s limited attention on the data points and sites that pose the greatest risk to trial integrity, rather than wasting time on low-value verification.
- Agentic AI for Workflow Automation: The next frontier is Agentic AI, which acts like an autonomous digital teammate. An AI agent can be assigned a complex workflow, such as managing the site initiation visit scheduling process across dozens of sites and time zones. It can handle the back-and-forth communication, find mutually available times, and escalate to a human only when a problem arises, eliminating countless hours of unproductive administrative downtime.
Human-only clinical development has hit a ceiling of complexity and cost. AI provides the scalable intelligence and automation we desperately need to break through it.
Integrate or Stagnate: Why a Single Source of Truth Is Non-Negotiable
If your medical trial software can’t communicate seamlessly with your other critical systems, you’re building your research on a foundation of sand. Modern trials generate a torrent of data from EDC, ePRO, central labs, local labs, EHRs, and wearables. Without robust integration, you will spend more time and resources reconciling data than analyzing it.
A unified platform connects your CTMS, EDC, ePRO, eTMF, and other tools into one coherent ecosystem. The backbone of this ecosystem is a set of robust, well-documented Application Programming Interfaces (APIs). This API-first approach allows data to flow seamlessly between systems, creating a single source of truth for all trial information. This dramatically reduces manual data entry, improves data quality by eliminating transcription errors, and enables real-time, cross-functional oversight for all stakeholders. This is the foundation of an effective Clinical Trial Data Integration Complete Guide.
Data Integrity and Compliance: The Unbreakable Foundation of Trust
In clinical trials, there is no middle ground on compliance. Your medical trial software must be built on a foundation of strict, demonstrable adherence to global regulations. Our guide on Clinical Trial Patient Data explains why these regulations are so critical. Key standards include:
- 21 CFR Part 11: This US FDA regulation governs the use of electronic records and electronic signatures. Your software must provide secure, computer-generated, time-stamped audit trails that record the who, what, when, and why of every action. Electronic signatures must be uniquely linked to an individual and applied to a specific record, ensuring non-repudiation.
- GDPR (General Data Protection Regulation): This EU regulation sets a global standard for data privacy. Your software must support core principles like “data minimization” (collecting only necessary data), “right to be forgotten” (securely deleting patient data upon request), and granular consent management, allowing patients to control how their data is used.
- HIPAA (Health Insurance Portability and Accountability Act): This US law mandates strict protections for Protected Health Information (PHI). Your software must feature technical safeguards like end-to-end data encryption (both in transit and at rest), role-based access controls to ensure users only see the data they need, and a willingness from the vendor to sign a Business Associate Agreement (BAA).
Your platform must be designed for future-proof compliance. Yet, a shocking 17% of organizations still have no plans to modernize their clinical trial systems. Industry reports consistently show that organizations with unified, modern clinical operations are not just more efficient—they are significantly more compliant. Relying on legacy platforms is not just outdated—it’s a dangerous gamble that will eventually lead to regulatory fines, rejected data, and invalidated research.
The Choice Is Clear: Modernize Your Trial Software or Fall Behind
Clinical trials are growing more complex, distributed, and data-intensive. The medical trial software you choose today will determine whether your organization leads or lags in this new landscape.
We’ve shown how outdated software creates operational bottlenecks, data silos, and compliance risks. The right unified platform solves these issues by integrating EDC, ePRO, eConsent, and analytics to streamline workflows and ensure data integrity.
The shift to decentralized and hybrid trials is permanent. Your software must support remote data collection, virtual visits, and wearables as standard features. The 10 essential capabilities we outlined are the new benchmark for high-performing platforms.
Looking ahead, the future is integrated and intelligent. AI is automating patient matching and predicting outcomes, while a single source of truth is essential for quality and oversight. Rock-solid compliance with standards like 21 CFR Part 11, GDPR, and HIPAA is non-negotiable.
For organizations handling complex, multi-omic data—spanning genomics, proteomics, and real-world evidence—standard software often falls short. At Lifebit, our next-generation federated AI platform is built for this challenge. It enables secure, real-time access to global biomedical data, with built-in AI/ML analytics and federated governance to power large-scale, compliant research.
The stakes are clear. Choosing the right medical trial software is about equipping your teams to work smarter, ensuring data quality, and accelerating the path to therapy. Don’t be among the 17% with no modernization plans. The technology is here. The benefits are proven. The choice is yours.
Learn how a unified, AI-driven platform can transform your clinical research.

