Beyond the Buzz: Demystifying Precision Health Data and Its Real-World Power

Why Precision Health Data is Changing Modern Medicine
Precision health data represents a fundamental shift from one-size-fits-all medicine to personalized care. Unlike traditional healthcare’s reliance on episodic visits and single lab tests, precision health integrates diverse, continuous data streams—including genomic, clinical, lifestyle, and environmental data—tracked longitudinally over time.
This multi-modal approach enables doctors to predict disease before symptoms appear, personalize treatments, and monitor health dynamically. As Canada’s $200 million precision health initiative shows, nations are investing heavily in large-scale genomic datasets to power AI-driven breakthroughs.
The promise is compelling: catching disease before it strikes through proactive, data-driven insights. However, the reality involves complex challenges around data integration, privacy, and equity.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. For over 15 years, I’ve focused on building platforms for secure, federated analysis of precision health data. My work in computational biology and AI helps organizations open up insights from siloed datasets without compromising privacy or compliance.
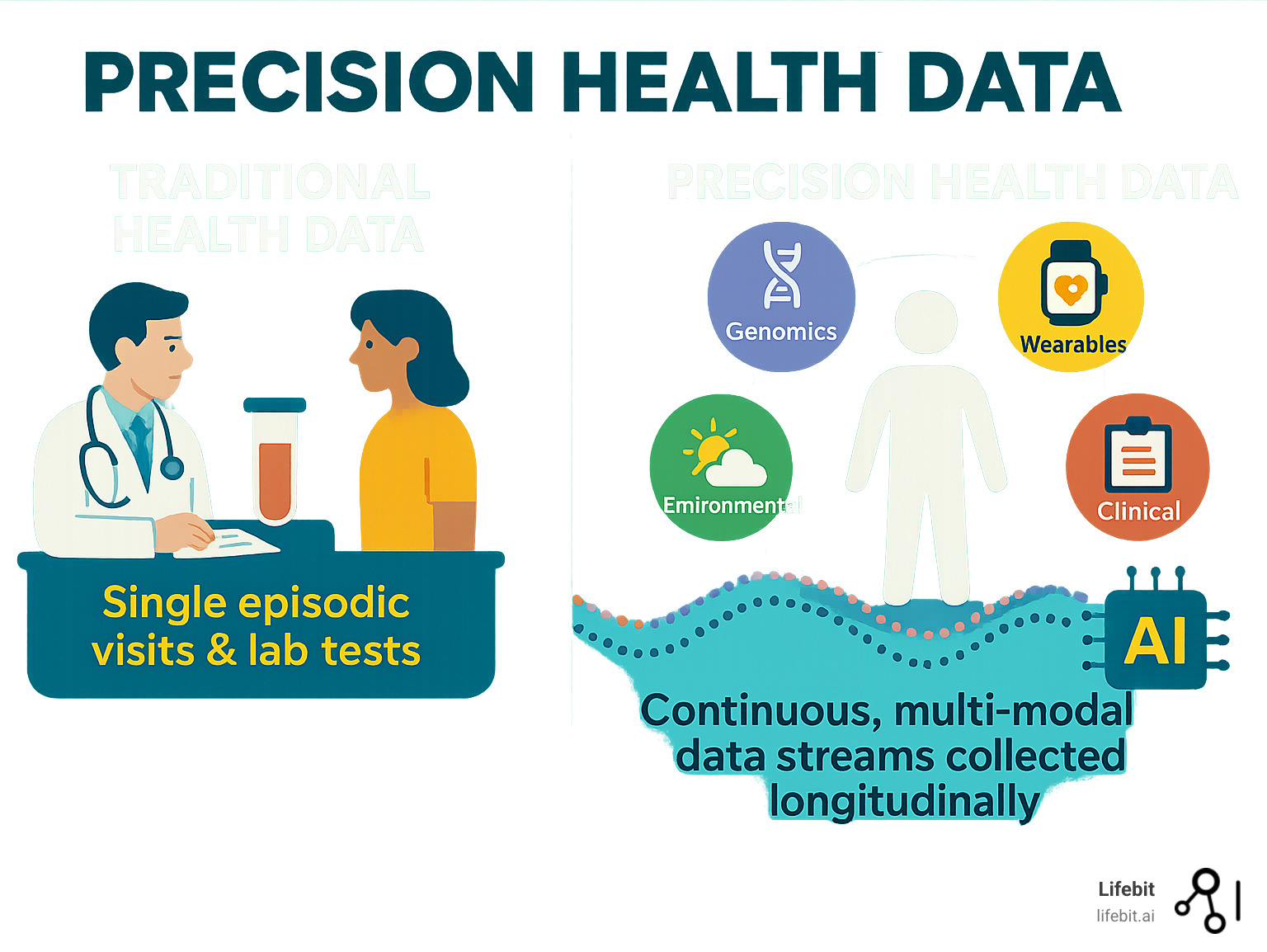
What is Precision Health Data? A Multi-Layered View of Human Health
Think of precision health data as creating a complete, living portrait of your health—one that captures not just a moment in time, but how your body changes and responds. Unlike traditional medicine’s single blood tests or annual checkups, precision health weaves together multiple data streams to understand your unique biological story.
This approach relies on multi-modal data from many different sources, often capturing thousands of measurements simultaneously (high-dimensional data). What makes it truly powerful is longitudinal analysis—tracking this information over months and years to spot patterns invisible in a single snapshot.
The Critical Data Modalities
Key data modalities each add a crucial layer. Genomics, often from whole-genome sequencing (WGS), forms the foundation. Electronic health records (EHRs) and medical imaging (MRI, CT scans) document your medical journey. Wearables and mobile health devices capture daily lifestyle rhythms, while microbiome analysis reveals the impact of gut health. Environmental data tracks external influences, and pathogen genomics is vital for monitoring public health crises like COVID-19. All this information requires secure, scalable analysis pipelines to manage its complexity.
Why Longitudinal Data is a Game-Changer
Instead of looking at health like a photograph, longitudinal data creates a movie. This continuous monitoring is revolutionizing how we understand health and disease.
A groundbreaking scientific study on longitudinal big data following 109 people for up to 8 years found over 67 clinically actionable health findings missed by traditional tests. This demonstrates the power of continuous monitoring.
For example, the study showed how early detection of risk works in practice. Two participants showed diabetic-level glucose patterns on continuous monitors despite normal lab tests, and another had rising inflammatory markers a full year before a lymphoma diagnosis. This dynamic health monitoring allows for precise tracking of disease progression and real-time treatment adjustments.
This approach enables detecting disease risk earlier than ever before. By spotting subtle changes before symptoms appear, precision health shifts medicine from reactive treatment to proactive prevention, catching problems while they are still manageable.
Building Representative Datasets: The Foundation for Equitable AI
The power of precision health data lies not just in its depth but also its breadth. To build effective AI, we need to capture the full diversity of human health. Without representative datasets, even sophisticated AI can amplify existing health disparities, making data quality, data scale, and algorithmic fairness paramount.
Why Scale and Diversity Matter for Precision Health Data
An AI model trained only on one population group may fail to detect risks in others—a technical and moral failure. That’s why we need massive, diverse datasets to train generalizable AI models that work for everyone.
National initiatives recognize this. Canada’s $200 million precision health initiative is building a genomic dataset of over 100,000 individuals representing its multicultural population. Brazil is leveraging a 100 million-person cohort for public health research, while the MSSNG database for autism research has grown to 13,500 whole-genome sequences through global collaboration. As the UNICEF report on precision health for children highlights, we must ensure these innovations serve our most vulnerable populations.
Ensuring Equity and Indigenous Data Sovereignty
Building representative datasets requires genuine community engagement, especially with Indigenous communities. Indigenous Data Sovereignty, guided by principles like OCAP® (Ownership, Control, Access, and Possession), ensures communities maintain control over their data.
Initiatives like the Pan-Canadian Genome Library show how to respect data governance while enabling research. This requires inclusive recruitment strategies, transparent communication, and benefit sharing models that deliver tangible returns to participating communities. It’s also vital to ensure access for rural and remote areas so precision health isn’t a privilege for urban centers.
Identifying and Mitigating Bias in Precision Health Data
Bias can enter precision health data at any stage, from biased EHRs to skewed recruitment. Algorithmic auditing is therefore essential to check AI models for biased outputs and embed fairness metrics into development. Monitoring for inequitable outcomes helps track how interventions affect different groups, allowing for course correction.
Prospective validation in diverse, real-world settings is crucial before widespread deployment. Furthermore, reporting checklists for precision health research, like the BePRECISE guidelines, promote transparency and help identify potential biases. Getting this right isn’t just about better algorithms—it’s about building a more just healthcare system.
The Technical Ecosystem: Securely Connecting and Analyzing Global Data
Building a world where precision health data can truly transform medicine requires a technical ecosystem that can handle massive, sensitive datasets securely. The challenge is connecting global data—genomic sequences, EHRs, wearable data—while protecting patient privacy and respecting national data sovereignty.

Centralized vs. Federated Networks: A Trade-off Analysis
A key decision is whether to centralize data or use federated networks. While centralized repositories gather all data in one place, they create a single point of failure and complicate compliance with international data laws.
| Feature | Centralized Repositories | Federated Networks |
|---|---|---|
| Privacy | High risk of single point of failure/breach; data aggregation increases re-identification risk. | Data remains at source; insights derived without moving data, enhancing privacy. |
| Scalability | Can become a bottleneck for data transfer and storage; complex to scale globally. | Inherently scalable; new data sources can join network without massive transfers. |
| Compliance | Difficult to meet varied international/regional data residency laws. | Easier to comply with local regulations and data sovereignty principles. |
| Data Sovereignty | Data is moved away from original jurisdiction/ownership. | Data remains under the control of its original custodian. |
| Utility | Easier for single-site analysis; full dataset available. | Requires sophisticated infrastructure to “move questions to the data”; insights are aggregated, not raw data. |
For precision health data, federated networks are a compelling solution. Analysis is moved to the data, not the other way around. This approach relies on privacy-preserving technologies like federated learning, where AI models train on distributed data without it ever leaving its source. Other methods include secure enclaves, de-identification, and differential privacy.
At Lifebit, our federated analysis platform enables this approach, allowing insights to be derived without compromising data location or control.
The Role of Interoperability and Open Standards
Healthcare data comes in many formats, creating silos. The solution is open standards that act as universal translators. FHIR (Fast Healthcare Interoperability Resources) connects clinical systems, common data models create shared research vocabularies, and GA4GH standards enable responsible genomic data sharing.
These standards, along with FAIR data principles (Findable, Accessible, Interoperable, Reusable), create a foundation for collaboration. The Canadian Platform for AI in Health exemplifies this, accelerating AI tools through standards-compliant federated networks. The goal is to avoid vendor lock-in and foster an ecosystem where innovation can flourish.
Infrastructure and Workforce Requirements
This ecosystem demands significant investment. On the technical side, we need high-throughput sequencing centers and secure cloud infrastructure with high-performance computing to handle petabytes of data.
The human element is also critical. We need data stewardship roles for ethical management, clinical informatics experts to bridge healthcare and technology, and MLOps specialists to ensure AI models work reliably in clinical settings. Perhaps most importantly, reproducible analysis workflows and standardized methods are needed to ensure scientific findings can be verified and built upon by others.
High-Impact Applications: Turning Data into Clinical Reality
The true test of precision health data is its impact on patient outcomes. We are now seeing this data transform how doctors diagnose diseases, predict risks, and improve population health.
Changing Diagnosis and Treatment in Key Disease Areas
The impact is dramatic in complex fields. In precision oncology, genomic profiling of tumors guides the selection of targeted therapies that are more effective and less toxic. For rare diseases, whole-genome sequencing is ending the “diagnostic odyssey” by quickly identifying genetic causes. The expansion of resources like Autism Speaks’ MSSNG database to 13,500 genomes is helping researchers find autism subtypes for personalized approaches.
Diabetes is another example. Precision health data reveals that “type 2 diabetes” is actually several distinct conditions, enabling personalized management. In infectious disease surveillance, combining pathogen genomics with clinical data allows for real-time outbreak tracking and is essential for pandemic preparedness.
The Future of Proactive Health and Public Health
The most exciting shift is from reactive to proactive care. Longitudinal multi-omics monitoring can detect biological changes years before symptoms appear, signaling risks for cancer or diabetes. Wearable data from continuous glucose monitors, sleep trackers, and heart rate sensors provides a constant stream of health signals, alerting patients and doctors to emerging issues.
A key challenge is returning actionable results to participants in large-scale studies, which requires robust systems for genetic counseling and clinical follow-up. At a population level, wastewater surveillance offers an early warning system for community-wide outbreaks, while large-scale population cohort studies, like Brazil’s 100 million-person cohort, provide unprecedented insights into disease patterns.
The Roadmap to Clinical Deployment
Moving from research to routine care involves navigating several challenges. The path from data generation to clinical use requires clear workflows for integrating insights into medical practice. Regulatory milestones and reimbursement policy must adapt to AI-driven diagnostics and preventative care.
Public-private partnerships are essential. Genome Canada’s investments, which have leveraged over $1 billion in co-funding, show how collaboration can build sustainable ecosystems. National and regional genomics centres act as crucial hubs for this work.
At Lifebit, our federated analysis platform is designed to bridge this gap, enabling secure access to global data to power the next generation of high-impact applications.
Frequently Asked Questions about Precision Health Data
When organizations consider precision health data initiatives, questions about privacy, risks, and governance are common. Here’s how we address them.
How is patient privacy protected when using precision health data?
Protecting privacy is about building trust. We use advanced privacy-preserving technologies to derive insights while keeping data secure.
Federated learning is a key breakthrough. Instead of moving patient data, we bring the analysis to the data. AI models learn from distributed datasets, but only aggregated insights—never raw patient information—are shared.
This is layered with other techniques like de-identification and secure enclaves (digital vaults for computation). Crucially, these technologies are supported by robust governance frameworks, including data access committees, audits, and dynamic consent models that give patients ongoing control. For Indigenous communities, data sovereignty principles ensure control remains with the rightful owners.
What are the biggest risks, and how are they managed?
Working with precision health data involves real risks that require comprehensive safeguards.
- Re-identification risk: While small, the risk of re-identifying individuals from anonymized data exists. We mitigate this with strict access controls, secure computing environments, and differential privacy techniques.
- Algorithmic harm: AI models trained on biased data can perpetuate health disparities. We address this with rigorous bias detection, continuous monitoring across demographic groups, and regular audits.
- Misuse by insurers or employers: Strong legal protections, like genetic non-discrimination laws, are the first line of defense. We reinforce these with strict data use agreements and comprehensive audit logs to create technical and legal accountability.
Ethical oversight boards provide independent review to ensure the benefits of any research justify the risks.
How can large-scale data initiatives respect data sovereignty?
Data sovereignty is fundamental for building trust and enabling global collaboration, especially when working across provinces or with Indigenous communities.
Federated networks are the solution. They keep data within its original jurisdiction, satisfying local privacy laws and governance requirements. Researchers send analytical queries to the data rather than moving the data itself. This makes cross-border research possible without data movement.
For Indigenous communities, frameworks like OCAP® (Ownership, Control, Access, and Possession) ensure they retain authority over their data, honoring self-determination. By aligning with global standards like those from GA4GH, we create interoperability without sacrificing sovereignty. This inclusive approach encourages participation, helping build the diverse datasets needed to serve all populations.
Conclusion
The future of medicine is data-driven, with precision health data at its heart. This approach moves us from reactive, one-size-fits-all healthcare to a more powerful model: proactive, predictive, and truly personalized care. By combining genomic insights with real-world evidence, we create a complete picture of human health that was previously impossible.
Building this future rests on three pillars:
Collaboration: Major breakthroughs, like Canada’s $200 million precision health initiative, arise from public-private partnerships that pool resources, expertise, and infrastructure.
Equity: Precision health must benefit everyone. This requires engaging diverse populations, respecting Indigenous data sovereignty through frameworks like OCAP®, and building globally representative datasets.
Trust: The foundation of it all. Privacy-preserving technologies like federated learning and secure enclaves are trust-building tools that give people the confidence to participate in research.
Measuring our progress will also change. Instead of only treating disease, we will measure our ability to prevent disease before symptoms emerge, shorten diagnostic timelines, and ensure new therapies reach all populations equitably.
At Lifebit, our mission is to make this vision a reality. Our federated AI platform enables secure, real-time access to global biomedical data without compromising privacy. By allowing researchers to “move questions to the data,” we are building the infrastructure for truly global precision health research.
The road ahead is challenging but promising. The foundation we build today with precision health data will determine how quickly we can turn scientific breakthroughs into better health outcomes for everyone.

