Unlocking Data Secrets Safely with Trusted Research Environments

Why Trusted Research Environments Are Essential for Modern Healthcare
A trusted research environment is a secure computing platform that allows approved researchers to access and analyze sensitive health data without ever moving it outside a controlled digital space. Think of it as a high-security reference library where valuable datasets and analytical tools live together, accessible only to verified users who follow strict protocols.
Key Components of a Trusted Research Environment:
– Secure Access Control – Multi-factor authentication and role-based permissions
– Data Protection – Encryption, de-identification, and audit trails
– Controlled Analytics – Pre-approved tools and monitored outputs
– Compliance Framework – GDPR, HIPAA, and regulatory adherence
– Collaborative Workspace – Multiple researchers can work together safely
The need for trusted research environments has exploded as healthcare organizations struggle with data silos, lengthy approval processes, and regulatory bottlenecks. Traditional data sharing often takes six months or longer for approvals, while biobanks now routinely manage petabytes of genomic data.
During the COVID-19 pandemic, trusted research environments proved their worth by enabling rapid collaboration on critical research. The UK’s Secure Research Service alone supports over 600 active research projects, demonstrating how these platforms can accelerate findy while maintaining the highest security standards.

What is a Trusted Research Environment?
A trusted research environment (TRE) is a highly secure digital workspace where approved researchers can access and analyze sensitive datasets without ever moving that data outside the protected environment. Also called Safe Havens or Secure Data Environments, these platforms have become the backbone of modern biomedical research.
The concept is built on a simple idea: instead of copying sensitive data and sending it to researchers around the world, we bring the researchers to where the data lives safely. This fundamental shift has transformed how we handle everything from patient records to genetic sequences.
TREs first emerged in the UK around 2010, born from the recognition that traditional data sharing was both too risky and too slow for modern research needs. What started as a solution to protect NHS patient data has now become the global gold standard for secure biomedical collaboration.
TRE as a Secure “Reference Library”
Just as you might visit a special collections library to study rare books that never leave the premises, researchers log into a TRE to work with sensitive datasets that remain permanently within the secure environment.
In this digital research library, data never leaves the building. Researchers bring their questions and analytical methods, but they can’t download raw datasets to their laptops or email files to colleagues. Instead, they work entirely within the controlled environment, collaborating with other approved researchers on the same valuable datasets.
This “no-data-leave principle” is what makes TREs so powerful. All research activities are carefully logged and monitored. Only aggregated, anonymized results can be exported after thorough review. Multiple research teams can work simultaneously on the same datasets without anyone ever possessing their own copy.
Evolution and Global Adoption
Europe has led the charge in TRE development, with the UK pioneering large-scale implementations through organizations like NHS Digital and Genomics England. The COVID-19 pandemic became a turning point for global TRE adoption, as researchers worldwide needed rapid access to sensitive health data.
Today, TREs are expanding into biopharma and commercial applications. Pharmaceutical companies are finding that TREs give them access to valuable real-world evidence while maintaining regulatory compliance and building public trust.
Core Principles: Five (and Seven) Safes Framework
The Five Safes framework, originally developed by the UK’s Office for National Statistics, provides the blueprint for building secure trusted research environments. This framework balances open science with ironclad security through seven interconnected principles.
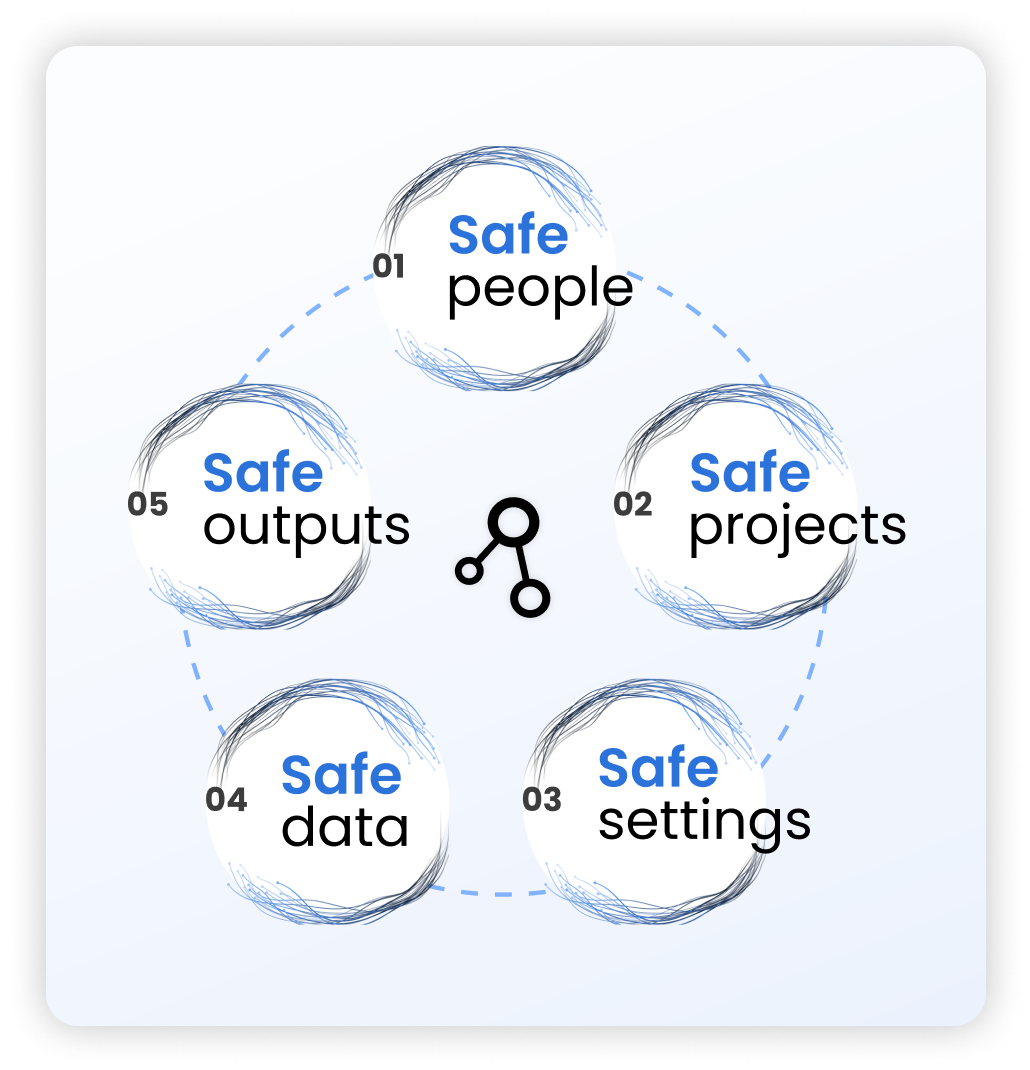
Safe People forms the human foundation. 85% of TREs require researchers to complete mandatory training covering information governance, GDPR, privacy, ethics, and security. Another 79% require signed agreements promising not to misuse the environment or data.
Safe Projects ensures only research with genuine scientific merit gets approved. Independent review boards evaluate every research proposal, including ethical review and data protection impact assessments.
Safe Settings covers the technical infrastructure. 64% of TREs use private on-premises cloud infrastructure, while multi-factor authentication and VPN access have become standard requirements.
Safe Data focuses on protecting information through de-identification and anonymization. Data undergoes pseudonymization with k-anonymity thresholds (typically k≥5) preventing re-identification of individuals.
Safe Outputs controls what information can leave the secure environment. All TREs allow export of aggregate-level data with minimum cell sizes, though 73% still rely on human analysts rather than automated tools.
The framework has evolved to include Safe Computing (adequate computational resources) and Safe Return (long-term lifecycle management of research outputs).
Legal & Ethical Compliance in TREs
GDPR compliance sits at the heart of any TRE handling European data, demanding explicit consent mechanisms, data protection impact assessments, and the right to erasure. Key compliance elements include patient consent management, ethics board oversight, audit trail maintenance, and cross-border data transfer compliance.
Roles and Responsibilities Inside a TRE
Researchers complete mandatory training, follow security protocols, and submit outputs for review. Data controllers manage access permissions and ensure compliance with legal requirements. IT security teams maintain technical infrastructure, handle incident response, and manage user authentication systems.
Security Architecture & Workflow in a Trusted Research Environment
Building a secure trusted research environment relies on multiple layers of protection working together seamlessly. Modern TREs use a “zero-trust” approach, where every request gets verified and every action gets checked.
The network security foundation starts with VPN-only access and IP whitelisting. The network is segmented into isolated zones, so breaches in one area can’t spread. Identity and access management requires multi-factor authentication and role-based access controls.
Data protection encrypts everything twice – at rest and in transit. Encryption keys are protected by specialized hardware security modules and rotated regularly. Monitoring systems log every action and can spot unusual patterns indicating potential security issues.
| Feature | On-Premises TRE | Cloud-Based TRE |
|---|---|---|
| Control | Full infrastructure control | Shared responsibility model |
| Scalability | Limited by hardware capacity | Elastic scaling on demand |
| Compliance | Direct audit and certification | Cloud provider certifications |
| Cost Model | High upfront, predictable ongoing | Pay-as-you-use, variable |
Data Access: Import, Analysis & Export Controls
Data entering a trusted research environment undergoes risk assessment and quality control checks. Personal identifiers get removed through pseudonymization before researchers access the data.
During analysis, researchers work in isolated virtual environments with no internet access. The export process requires all outputs to pass through an “airlock” where results get reviewed before release. Only aggregate-level outputs can leave, with minimum cell sizes of at least 5 people in any reported group.
Currently, 73% of TREs rely on human experts to review outputs manually, though automated tools are becoming more common.
More info about Streamlining Data Analysis
Technical Safeguards & Certifications
ISO 27001 certification has become the gold standard for information security management. SOC 2 Type II and HITRUST CSF provide additional healthcare-specific validation.
64% of TREs currently use private on-premises infrastructure for complete control, while 21% have moved to public cloud platforms for scalability and cost-effectiveness. Real-time monitoring using AI-powered security tools can detect unusual patterns and alert security teams to potential issues before they become serious problems.
Powering Next-Generation Research: AI, Big Data & Federated Learning
Today’s trusted research environments handle massive AI pipelines, process petabytes of genomic data, and enable global collaboration without sharing raw data. A single human genome generates 750 MB of data – imagine analyzing genetic patterns across millions of people or training AI models for drug response prediction.
Modern TREs provide sophisticated AI/ML pipeline support with GPU clusters, containerized workflows, and automated parameter tuning. Big data analytics capabilities include distributed computing frameworks and real-time streaming processors.
Federated learning infrastructure enables multiple institutions to train AI models together without data leaving their secure environments. This approach allows collaborative research while maintaining strict privacy controls.
The challenge of safely exporting AI models is particularly complex. Unlike simple statistics, AI models can potentially leak information through membership inference attacks or model inversion techniques. Differential privacy has emerged as a key solution, adding calibrated noise to protect individual privacy while preserving overall patterns.
Latest research on privacy attacks
Case Studies & Real-World Examples
SAIL Databank in Wales provides anonymized health records for the entire Welsh population. UK’s Secure Research Service supports over 600 active research projects and provided COVID-19 researchers with access to records for 54 million people.
OpenSAFELY publishes all analysis code openly while keeping data secure, building public trust through transparency. Genomics England manages secure access to 100,000 complete human genome sequences with clinical data, supporting both academic research and commercial drug development.
Collaboration Across Institutions
Modern trusted research environments enable multi-site studies through secure portals, data-in-place analytics, and federated identity management. Researchers can access approved datasets across multiple institutions while each organization maintains complete control over their data.
Our federated architecture enables collaboration at the analysis level rather than the data level, changing rare disease research where no single hospital sees enough patients for meaningful conclusions.
Challenges, Limitations & Future Directions
While trusted research environments have revolutionized secure data analysis, they face growing pains. The biggest challenge is that manual processes still dominate – 73% of TREs rely on humans to check every output, creating bottlenecks that slow research.
Scalability presents another challenge. Many TREs were built as secure but expensive fortress computers that struggle to expand when research teams need more computing power. High infrastructure costs can lock out smaller institutions.
The data variety problem affects TREs designed for neat spreadsheets but now handling medical images, genetic sequences, and sensor data. AI model export challenges create tension between advancing AI research and protecting privacy, while public trust remains fragile despite robust security measures.
Innovations on the Horizon
Homomorphic encryption enables analyzing data while it stays completely encrypted. Secure multiparty computation allows multiple organizations to jointly analyze combined data without any single party seeing the complete picture. Synthetic data generation creates realistic practice datasets without containing actual patient information.
Recommendations for Continuous Improvement
User experience needs improvement – current interfaces often feel designed by security experts for other security experts. Federated ecosystem development requires standardized protocols and shared governance frameworks. Automated disclosure control can solve manual bottlenecks through AI-powered privacy risk assessment. Global standards alignment could enable truly international collaborative research.

Frequently Asked Questions about Trusted Research Environments
What kinds of data can be analysed in a TRE?
Trusted research environments handle any sensitive or personally identifiable data. Health and clinical data includes electronic health records, clinical trials, and prescription records. Genomic data ranges from whole genome sequences to GWAS datasets. Medical imaging encompasses pathology slides and radiology scans, while administrative and social data includes insurance claims and education records.
The key requirement is sensitivity level, not data format – whether structured databases or unstructured images and audio files.
How do TREs prevent re-identification of individuals?
Technical safeguards remove direct identifiers through de-identification and pseudonymization. Statistical disclosure control uses k-anonymity principles ensuring each record is indistinguishable from at least 5-10 others. Procedural controls enforce minimum cell sizes and manual output review. Environmental controls prevent internet access and monitor all sessions.
These multiple layers work together to create robust protection while preserving research value.
Can AI models be safely exported from a TRE?
AI models present unique privacy challenges through membership inference attacks, model inversion techniques, and property inference attacks. Current approaches vary from allowing only aggregate statistics to requiring extensive privacy analysis. Federated learning offers a promising solution by enabling distributed training without data leaving home institutions. Best practices are evolving with differential privacy techniques and case-by-case risk evaluation becoming standard.
Conclusion
The journey through trusted research environments reveals a fundamental shift in healthcare research – from copying and distributing sensitive datasets to secure, collaborative access that protects data while open uping its potential.
TREs supporting over 600 active research projects, rapid COVID-19 breakthroughs, and growing pharmaceutical adoption demonstrate this isn’t just a trend – it’s the new foundation for biomedical research. The trusted research environment approach solves decades-old problems: no more six-month approval waits, compliance worries, or isolated research when collaboration could accelerate breakthroughs.
At Lifebit, our federated AI platform brings together everything modern TREs need – from secure multi-party computation to AI-powered privacy protection. Our platform components work seamlessly: the Trusted Research Environment for secure analysis, the Trusted Data Lakehouse for scalable data management, and R.E.A.L. (Real-time Evidence & Analytics Layer) for immediate insights.
TREs are democratizing access to powerful datasets. Rare disease researchers can access population-scale genomic data, public health agencies can track outbreaks in real-time, and pharmaceutical companies can generate real-world evidence without compromising patient privacy.
Challenges remain – better automation, user-friendly interfaces, and AI model export solutions. But these are opportunities for innovation. The TRE ecosystem evolves rapidly, and each solved challenge opens new research collaboration possibilities.
The future I envision has trusted research environments as common as email – essential infrastructure researchers use without thinking about complex security protections running behind the scenes. Data will stay where it belongs, researchers will collaborate freely across boundaries, and breakthrough findies will happen faster than ever.
Whether developing life-saving treatments, tracking public health trends, or uncovering genetic disease bases, trusted research environments offer a path forward that puts both innovation and responsibility first. The future of healthcare research is federated, secure, and collaborative – and it’s being built in TREs today.

