Decentralized Clinical Trial Model: 1 Bright Future
The Game-Changing Shift That’s Changing Medical Research
The decentralized clinical trial model is revolutionizing medical research by bringing studies directly to patients, eliminating the need to travel to traditional sites. This patient-centric approach uses digital health technologies to conduct trials remotely, making participation more accessible and convenient for diverse populations.
Quick Answer: What is the Decentralized Clinical Trial Model?
- Core concept: Clinical trials where some or all activities happen outside traditional research sites.
- Key locations: Patient homes, local healthcare facilities, community pharmacies.
- Main technologies: Telemedicine, wearable devices, mobile apps, electronic consent.
- Primary benefit: Removes geographic and logistical barriers to trial participation.
- Result: Higher patient retention, greater diversity, and faster recruitment.
The shift toward decentralized trials is a fundamental reimagining of clinical research, accelerated by the COVID-19 pandemic. Traditional trials often required a two-hour journey to study sites, a significant barrier for many. Remote methods have proven they can maintain scientific rigor while dramatically improving the patient experience.
The impact is significant: patient-centric trials recruit participants four months faster and show a 19% higher likelihood of bringing drugs to market. For oncology, that success rate jumps to 32% higher.
As CEO and Co-founder of Lifebit, I’ve seen how this model enables secure, federated access to diverse patient data. The future of research lies in bringing advanced analytics to where data lives, a principle at the core of our genomics platforms.
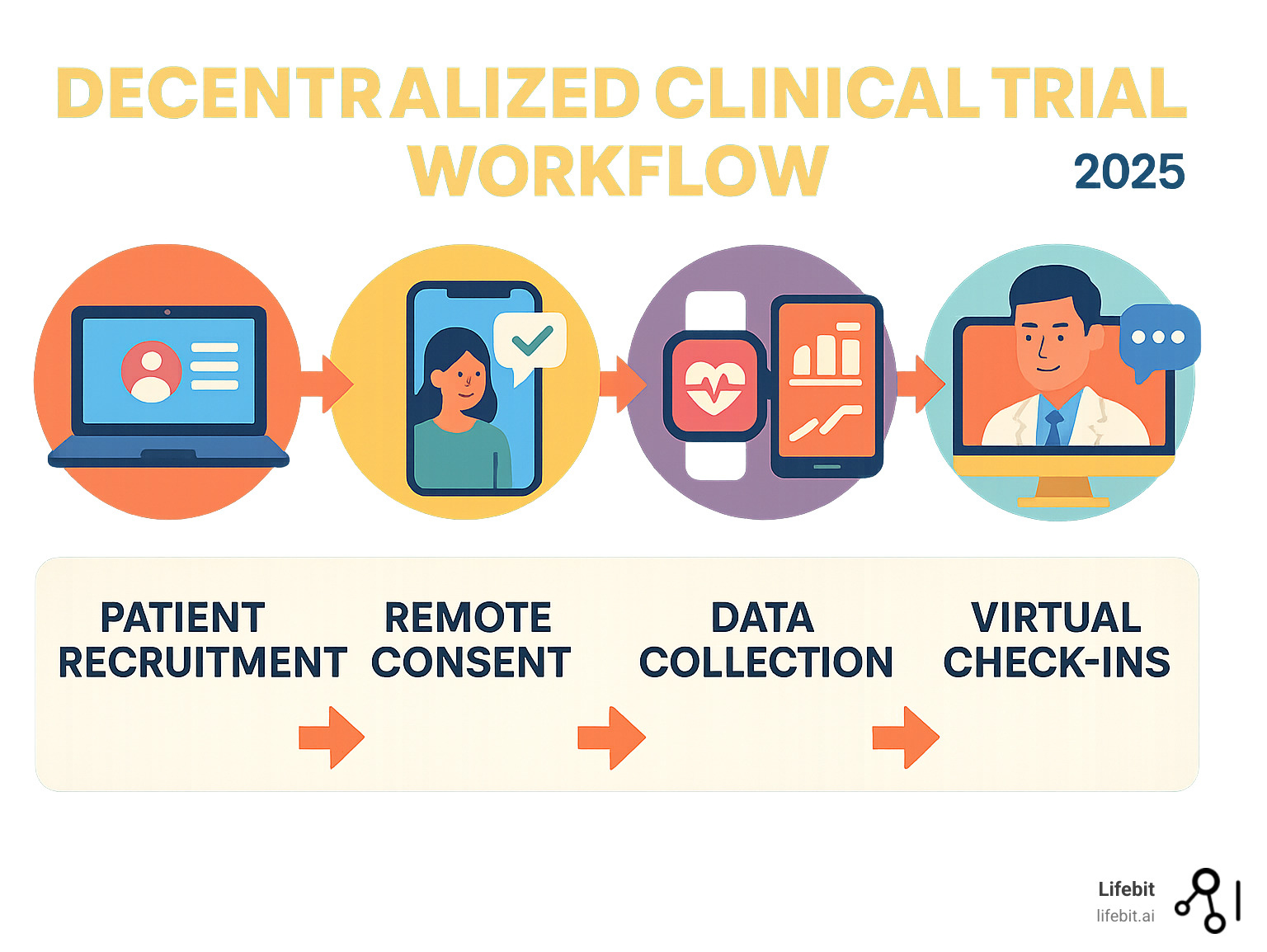
What is a Decentralized Clinical Trial? The Shift from Site-Centric to Patient-Centric

In a traditional clinical trial, participants often travel long distances to a central research site. This creates a significant participant burden, which includes not only taking time off work but also arranging childcare, incurring costs for travel and accommodation, and enduring the physical and emotional stress of travel, especially when ill. For many, this burden makes participation impossible. The decentralized clinical trial model flips this approach by bringing the trial to the patient.
The Federal Drug Administration (FDA) definition describes a decentralized clinical trial (DCT) as one where “some or all of the trial-related activities occur at locations other than traditional clinical trial sites.” In simple terms, research happens where it’s most convenient for participants—often in their own homes.
This concept isn’t new, but its adoption was accelerated by technology and the COVID-19 pandemic. Pfizer’s pioneering “virtual” trial in 2011, the REMOTE trial, was a landmark study that proved the viability of the model. It demonstrated that remote recruitment, consent, and data collection were feasible and that removing the travel burden could significantly improve patient engagement and retention. The model dismantles geographic barriers that historically excluded rural communities, people with mobility issues, and working parents from life-changing research.
Traditional vs. Decentralized Trials at a Glance
| Aspect | Traditional Clinical Trials | Decentralized Clinical Trials |
|---|---|---|
| Location | Centralized research sites only | Patient homes, local clinics, pharmacies |
| Patient Recruitment | Limited by geography and site capacity | Broader reach across diverse populations |
| Data Collection | In-person visits, paper forms | Digital tools, wearables, mobile apps |
| Monitoring | Periodic site visits by study monitors | Remote monitoring with real-time data |
| Patient Experience | High burden, frequent dropouts | Convenient, flexible, higher retention |
The Core Difference: Bringing the Trial to the Patient
The decentralized clinical trial model operates on a simple principle: meet patients where they are. The old site-centric model created an enormous participant burden, forcing patients to organize their lives around rigid study visit schedules. This often meant that the pool of participants was limited to those who lived near a major academic medical center and had the time and resources to participate.
The decentralized approach offers flexibility by unbundling trial activities from the central site. This can include:
- Home health visits: Nurses or phlebotomists conduct assessments, administer treatments, or collect biological samples (like blood or saliva) at a participant’s home.
- Local pharmacies and clinics: Routine procedures like blood draws, ECGs, or imaging are performed at convenient, familiar locations closer to the participant.
- Mobile research units: Specially equipped vans or temporary setups bring trial capabilities directly into communities, reaching underserved urban and rural populations.
- Direct-to-patient services: Study drugs, materials, and devices are shipped directly to the participant’s home, with clear instructions and support.
This participant convenience allows people to maintain their normal routines while contributing to medical research. By reducing the burden, DCTs dramatically improve study completion rates, leading to better data and faster answers to critical health questions.
The Core Benefits: Why This New Model is Gaining Momentum

The momentum behind the decentralized clinical trial model is backed by impressive results. By removing historical barriers, studies become faster, more diverse, and more successful. This transforms participation from a significant burden into a more integrated part of a patient’s healthcare journey.
Enhancing Patient Access, Diversity, and Retention
Traditional trial sites are often located in major urban academic centers, systematically excluding patients in rural areas or those without the means to travel. DCTs open doors to a much broader and more representative pool of participants. This dismantles geographic and socioeconomic barriers, as people no longer have to choose between participating in research and missing work or paying for travel.
This inclusivity is a critical ethical and scientific imperative. As the FDA guidance on enhancing diversity in clinical trials emphasizes, participation from varied demographic groups (including race, ethnicity, age, and gender) is crucial for developing treatments that are safe and effective for everyone. The results are clear: one study by Pfizer found that decentralized approaches achieved completion rates of 90%, far above the industry average, which often struggles with dropout rates of 30% or more. For rare disease studies, where every participant is precious, this model is game-changing, as it can connect a geographically dispersed patient population that would be impossible to assemble at a single site.
Accelerating Research and Improving Outcomes
The decentralized clinical trial model makes research more efficient and successful. With the cost of bringing a new drug to market estimated in the billions, any delay can have massive financial consequences. The data is compelling:
- Faster Recruitment: Studies on patient-centric technology efficiency show that patient-centric trials recruit participants in four months on average, compared to seven for traditional methods. This three-month acceleration can save millions of dollars and get vital treatments to the public sooner.
- Higher Success Rates: Trials incorporating decentralized elements are 19% more likely to bring drugs to market. For oncology, a field with notoriously high failure rates, this jumps to a 32% higher success rate.
These improved outcomes stem from reduced site-specific inconsistencies and higher data quality. Continuous monitoring via digital tools provides rich, real-world evidence. For example, instead of a single blood pressure reading taken in a stressful clinic environment (which can be skewed by “white coat hypertension”), a wearable device can collect hundreds of readings throughout the day in the patient’s natural environment. This provides a far more accurate and holistic picture of a treatment’s effect, leading to stronger evidence and more reliable conclusions.
At Lifebit, our federated data approach aligns perfectly with the DCT model. By generating insights across institutions without moving sensitive data, we bring analysis to where the data lives, avoiding central bottlenecks and accelerating research.
The Technology Backbone of the Decentralized Clinical Trial Model
A sophisticated tech stack makes the decentralized clinical trial model possible. These digital health technologies (DHTs) are the bridges connecting patients to research teams, allowing data to flow seamlessly from daily life to data centers. While the backend systems are complex, they create a simple, intuitive experience for participants.
Essential Technologies Enabling DCTs
An effective DCT weaves together several key technologies:
- eConsent platforms: Allow participants to review study information and provide consent electronically at their own pace, often using interactive videos and secure video calls.
- Telemedicine and video conferencing: Serve as the backbone for remote interactions, enabling secure consultations between participants and research teams.
- Wearable devices and sensors: Collect continuous, objective data like activity levels, sleep patterns, and vital signs, providing insights impossible to capture in periodic clinic visits.
- eCOA/ePRO platforms: Capture patient-reported outcomes and experiences directly through digital questionnaires on smartphones or tablets, providing real-time feedback.
- Direct-to-patient logistics: Ensure study medications and materials are delivered safely and on time to participants’ homes.
- Centralized data platforms: Act as the digital nervous system, securely integrating and managing the vast amounts of data from diverse sources. Lifebit’s federated AI platform excels here, providing secure, real-time access to biomedical data while ensuring privacy and compliance.
Hybrid vs. Fully Remote: Choosing the Right Approach
The level of decentralization can be custom to the study’s needs.
Fully decentralized trials are entirely remote, with all activities from recruitment to data collection handled digitally. This is ideal for studies that can be monitored through wearables and apps.
Hybrid models, the most common approach, blend remote activities with essential in-person visits. A participant might visit a clinic for an initial screening or complex procedure, while all routine follow-ups happen remotely. This model is common in oncology, where initial infusions may require on-site monitoring.
The choice depends on the intervention’s complexity, safety requirements, and patient needs. The goal is to minimize participant burden while maintaining the highest standards of safety and data quality. Nearly every trial can benefit from incorporating some decentralized elements.
Navigating the Landscape: Key Challenges and Considerations
While the decentralized clinical trial model is transformative, it’s not without challenges. The shift requires careful planning and new strategies to address data integrity, regulatory compliance, patient safety, and potential technology and human barriers.
Ensuring Data Quality and Security
Moving data collection from a controlled clinic to a patient’s home introduces new variables. In a DCT, data may come from a consumer fitness tracker, a patient’s smartphone, or a medical-grade sensor. This demands robust systems for validation and monitoring. For instance, sponsors must decide whether to provision participants with standardized devices or adopt a “Bring Your Own Device” (BYOD) policy, each with its own complexities for ensuring data consistency and accuracy. According to scientific research on data integrity, a comprehensive data management plan (DMP) is essential to map how information from these diverse sources is collected, standardized, integrated, and quality-checked.
With sensitive health information traveling across networks, data privacy and cybersecurity are paramount. Sponsors must implement end-to-end encryption, secure cloud storage, and clear protocols to protect against data breaches, meeting stringent FDA and global privacy expectations.
At Lifebit, our federated AI platform is built to address these challenges. It enables secure, real-time access to global biomedical data by keeping information at its source, ensuring the highest standards of privacy and compliance.
Overcoming Implementation and Ethical Problems
Beyond technology, there are significant human and operational challenges to steer:
- The Digital Divide: Not everyone has reliable internet access or is comfortable with technology. To ensure inclusivity and avoid exacerbating health disparities, trials must provide devices, data plans, comprehensive training, and accessible, multi-language technical support.
- Investigator Oversight: The investigator’s role shifts from direct, in-person management to remote monitoring of a geographically dispersed group. They need new tools, dashboards, and skills to maintain proper oversight of patient safety and data quality, including clear protocols for managing remotely reported adverse events.
- Site and Staff Adoption: Clinical research coordinators and site staff are accustomed to traditional workflows. They require extensive training and support to become proficient with new digital platforms, remote communication techniques, and the logistics of coordinating home health visits and direct-to-patient shipments.
- Maintaining Human Connection: Building trust and rapport through a screen requires intentional effort. Strategies like regular, scheduled video check-ins, assigning a dedicated remote study coordinator, and ensuring technology is user-friendly are essential for patient comfort, engagement, and retention.
- Ethical Considerations: Institutional Review Boards (IRBs) and ethics committees must adapt their review processes. They face new questions about ensuring true informed consent remotely, protecting vulnerable populations in a home setting, and managing patient privacy with continuous data collection.
These challenges are solvable with thoughtful planning, robust platforms, and a steadfast commitment to putting patients first.
Regulatory Oversight and Stakeholder Roles
The decentralized clinical trial model requires close collaboration between regulatory bodies, sponsors, and investigators, all of whom are adapting to new ways of working. Regulators globally are actively shaping this transition, creating frameworks that protect patients while encouraging innovation.
FDA Guidance and the Regulatory Framework
The FDA has been forward-thinking in its support for DCTs. Key developments include:
- Draft DCT Guidance: In May 2023, the FDA released a comprehensive draft DCT guidance document. This practical roadmap defines decentralized elements and provides clear instructions on topics like trial design, remote data collection, safety monitoring plans, and the validation of digital health technologies.
- DHT Guidance: The FDA also published separate guidance on Digital Health Technologies (DHTs), outlining expectations for their use in collecting data for regulatory submissions.
- Legislative Support: The Food and Drug Omnibus Reform Act of 2022 included requirements for the FDA to advance the use of DHTs and DCTs, signaling that this model is a key part of the future of clinical research.
The FDA has also adapted its own methods, conducting remote regulatory assessments to oversee trials. Globally, the European Medicines Agency (EMA) and other bodies are developing similar frameworks. The EMA’s initiatives aim to harmonize regulations for multinational DCTs, addressing the complexities of cross-border data flows and differing healthcare systems.
The Evolving Roles of Stakeholders
The shift to a decentralized clinical trial model has transformed the day-to-day responsibilities of key stakeholders.
Sponsor responsibilities have expanded significantly. Technology selection and validation are paramount, as sponsors must choose and vet the digital tools used for remote data collection. Vendor management is also far more complex, requiring the orchestration of multiple partners, including technology platforms, home health service providers, and direct-to-patient logistics companies. Sponsors must develop robust data management plans for information flowing from hundreds or thousands of devices—a challenge where Lifebit’s federated AI platform excels by enabling secure, real-time access to distributed data.
For investigators, the core responsibility for patient safety and trial integrity remains, but its execution changes. Remote oversight requires new skills and tools. They now monitor patient progress through data dashboards and video calls, coordinating care across scattered locations. This necessitates new standard operating procedures (SOPs) for tasks like remote adverse event reporting and virtual patient consultations. The Clinical Trial Change Initiative (CTTI) provides valuable guidance and recommendations for all stakeholders navigating these new roles.
Finally, the patient’s role evolves from a passive subject to an active partner. By using digital tools to contribute data directly from their daily lives, patients become collaborators in the research process, providing real-time feedback that is crucial for the trial’s success.
The Future is Bright: What’s Next for Clinical Research?
The decentralized clinical trial model is the foundation for the future of medical research. Its compelling benefits—faster recruitment, better retention, and greater diversity—are making clinical research more accessible, efficient, and patient-focused than ever before.
The Integration of AI and Real-World Evidence
The most exciting developments for the decentralized clinical trial model lie at the intersection of artificial intelligence (AI) and real-world evidence (RWE). This combination is open uping new possibilities.
AI and machine learning are revolutionizing how we analyze the massive data streams from DCTs. AI algorithms can spot patterns in continuous data from wearables that humans might miss, identifying early treatment responses or safety signals. AI-driven patient recruitment can also scan large datasets to find eligible participants faster and more efficiently.
The integration of real-world data is equally transformative. By capturing how treatments work in a patient’s daily life, RWE provides a much richer picture of a therapy’s true effectiveness and safety. This leads to predictive analytics, where models can forecast how individuals might respond to treatments, paving the way for personalized medicine.
The Trial Innovation Network (TIN) has been instrumental in advancing these approaches, demonstrating the value of decentralized elements in improving efficiency and reducing participant burden.
At Lifebit, we are at the forefront of this change with our federated AI platform. We enable secure, real-time access to global biomedical and multi-omic data while maintaining strict privacy and compliance. Our platform components—including the Trusted Research Environment, Trusted Data Lakehouse, and R.E.A.L. (Real-time Evidence & Analytics Layer)—deliver the real-time insights and AI-driven safety surveillance needed for sophisticated DCTs.
The future is one of continuous evolution toward nuanced hybrid models, with a focus on patient empowerment. As technology and regulations mature, DCTs will become even more seamless and inclusive, accelerating the delivery of life-changing therapies to all.
Conclusion
The decentralized clinical trial model has fundamentally transformed medical research, evolving from a pandemic necessity to a complete reimagining of how studies should work. This shift is about putting patients first, proving that good science does not have to come at the expense of convenience and dignity. By bringing trials to patients, research becomes more inclusive, efficient, and successful.
The results are clear: the decentralized clinical trial model accelerates recruitment, increases diversity, and boosts the likelihood of bringing new therapies to market by nearly 20% (and even more for oncology). While challenges like data quality, the digital divide, and maintaining a human connection exist, the entire research ecosystem is adapting. The FDA is providing clear guidance, and sponsors and investigators are embracing new technologies and methods.
The future integration of AI and real-world evidence will make this model even more powerful, enabling richer insights and true personalized medicine.
At Lifebit, we are proud to drive this change. Our federated AI platform provides the secure, real-time access to global biomedical data that sophisticated decentralized trials require. We empower researchers with advanced analytics while upholding the highest standards of privacy and compliance.
The decentralized clinical trial model is more than a new methodology; it’s a return to patient-centered, accessible research. The future of clinical trials is here, and it’s more human than ever.
Learn more about secure data collaboration

