Why Understanding Drug Findy Platform Definitions Is Critical for Modern Healthcare
The drug findy platform definition has become essential knowledge for anyone navigating today’s pharmaceutical landscape. At its core, a drug findy platform is an integrated technological and methodological framework that streamlines the identification, development, and optimization of therapeutic compounds from initial target identification through preclinical candidate selection.
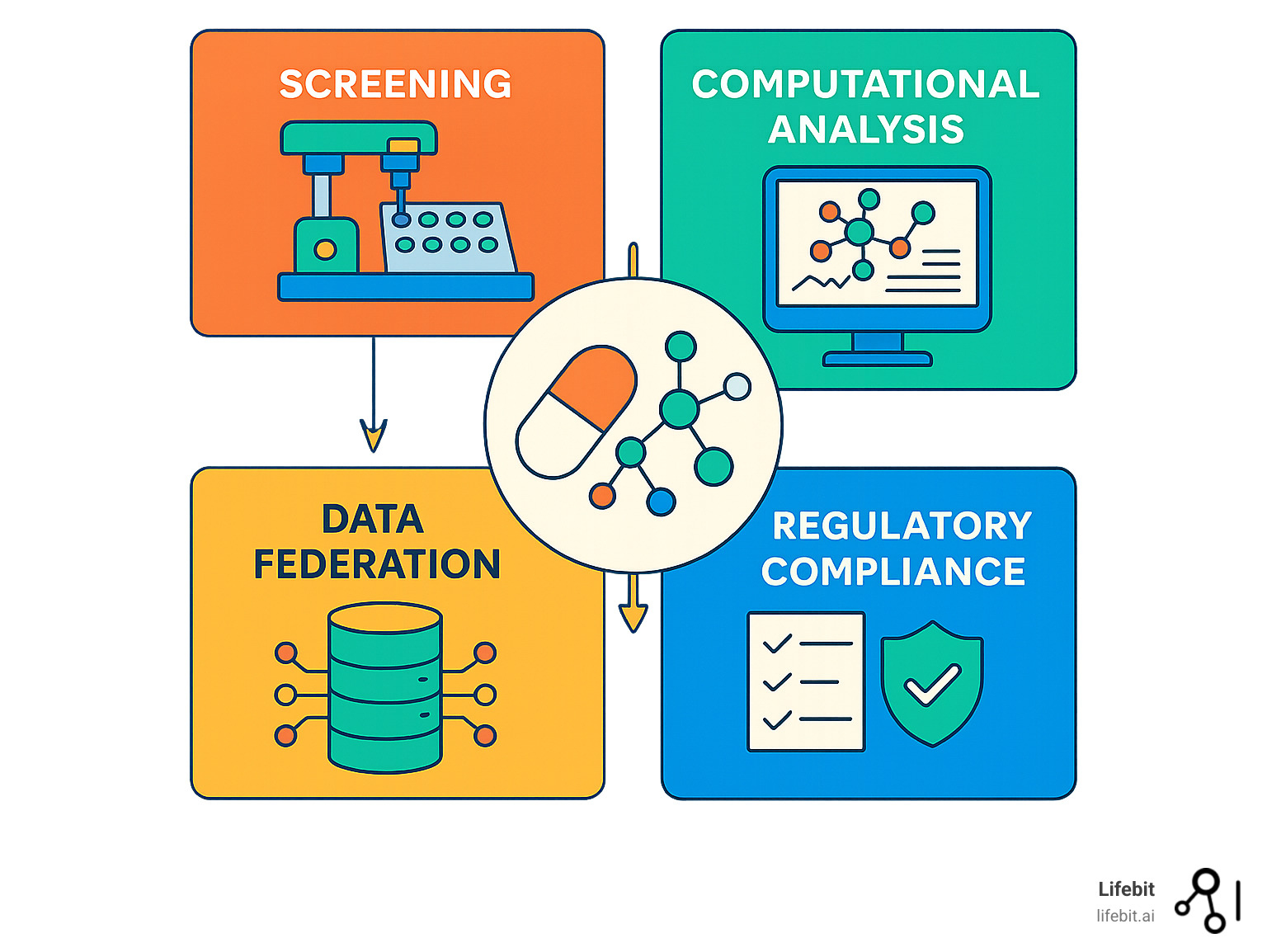
Key Components of Drug Findy Platforms:
- Screening Systems: High-throughput screening (HTS) capabilities testing millions of compounds
- Computational Tools: AI-driven modeling, virtual screening, and predictive analytics
- Data Integration: Multi-omic datasets, federated databases, and real-time analytics
- Automation Infrastructure: Robotics, assay miniaturization, and workflow orchestration
- Regulatory Frameworks: Compliance tools and standardized protocols
The stakes couldn’t be higher. Drug development takes 12-15 years and costs approximately $2.8 billion per approved medication, with failure rates exceeding 90%. Platform-driven approaches promise to dramatically reduce these timelines and costs by enabling systematic, data-driven decision-making across the entire findy pipeline.
Unlike standalone tools or individual technologies, these platforms create end-to-end workflows that connect target identification through lead optimization. They’re designed to break down data silos, accelerate screening processes, and provide the regulatory transparency that agencies like the FDA increasingly demand through initiatives like their Platform Technology Designation program.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent over 15 years building federated genomics and biomedical data platforms that power drug findy across secure, compliant environments. My experience developing computational biology tools and working with pharmaceutical organizations has given me deep insight into how a precise drug findy platform definition shapes successful therapeutic development.
Drug Findy Platform Definition: Core Concept
A drug findy platform definition encompasses far more than just laboratory equipment or software tools. It represents a comprehensive, modular architecture that orchestrates the entire target-to-clinic pathway through standardized, reproducible workflows.
Think of it as the difference between having a collection of kitchen appliances versus having a fully equipped commercial kitchen with integrated workflows, standardized recipes, and quality control systems. The platform approach transforms drug findy from a series of disconnected experiments into a systematic manufacturing process for new medicines.
The key distinction lies in integration and scalability. While individual tools might excel at specific tasks—like screening compounds or predicting molecular properties—platforms create synergies by connecting these capabilities into seamless workflows. This integration enables what we call “virtuous cycles of atoms and bits,” where computational predictions inform experimental design, and experimental results refine computational models in real-time.
Scientific Components of a Drug Findy Platform Definition
Modern drug findy platforms integrate several critical scientific components that work together to accelerate therapeutic development:
Target Identification and Validation Systems form the foundation, incorporating genomic databases, protein interaction networks, and disease pathway mapping. These systems help researchers identify which biological targets are most likely to yield effective therapies for specific conditions. Advanced platforms now integrate GWAS (Genome-Wide Association Studies) data, protein-protein interaction networks, and pathway enrichment analyses to prioritize targets with the highest probability of clinical success. For example, targets with genetic validation show a 2-3 fold higher success rate in clinical trials compared to those without genetic evidence.
Assay Development and Screening Infrastructure represents the experimental heart of the platform. High-throughput screening capabilities can test millions of compounds against biological targets, while automated assay development reduces the time from target identification to screening-ready protocols from months to weeks. Modern platforms incorporate multiple assay formats including biochemical assays, cell-based assays, and phenotypic screens. The integration of acoustic liquid handling and miniaturized assay formats has reduced reagent costs by up to 95% while improving data quality through reduced edge effects and improved statistical power.
Multi-omic Data Integration brings together genomics, proteomics, metabolomics, and clinical data to create comprehensive molecular portraits of diseases and drug responses. This integration is crucial for understanding complex biological systems and predicting drug efficacy and safety. Platforms now routinely integrate single-cell RNA sequencing data, spatial transcriptomics, and proteomics data to understand drug mechanisms at unprecedented resolution. The ability to correlate molecular signatures with clinical outcomes has become essential for predicting which patients will respond to specific treatments.
Federated Data Access enables secure collaboration across organizations while maintaining data privacy and compliance. Our experience at Lifebit has shown that federated approaches can increase dataset sizes by 10-100x compared to traditional centralized approaches, dramatically improving statistical power for drug findy analyses. Federated learning algorithms can now train AI models across multiple institutions without sharing raw data, enabling insights from datasets that would be impossible to combine using traditional approaches.
Machine Learning and AI Integration has become central to modern platform architectures. Deep learning models for molecular property prediction, generative models for compound design, and natural language processing for literature mining are now standard components. These AI systems can predict ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) properties with accuracy approaching experimental measurements, reducing the need for extensive early-stage testing.
Laboratory Automation and Robotics ensure reproducibility and scalability across screening campaigns. Modern platforms integrate liquid handling robots, automated incubators, plate readers, and analytical instruments into seamless workflows. The integration of machine vision systems for quality control and automated data capture has reduced human error rates by over 90% in high-throughput operations.
Why a Precise Drug Findy Platform Definition Matters
Regulatory clarity represents perhaps the most compelling reason for establishing precise platform definitions. The FDA’s new Platform Technology Designation program allows sponsors to leverage prior safety and manufacturing data across multiple products that share the same platform technology. This can reduce development timelines by months or years for subsequent products. Companies with designated platforms report 20-40% reductions in regulatory preparation time for follow-on products.
Scalability becomes critical as pharmaceutical organizations seek to improve their productivity. A well-defined platform can support multiple simultaneous programs, sharing infrastructure costs and accelerating time-to-market across entire therapeutic portfolios. The economics are compelling: while individual drug programs might require $50-100 million in findy costs, platform approaches can amortize infrastructure investments across dozens of programs, reducing per-program costs by 30-50%.
Data Standardization and FAIR Principles (Findable, Accessible, Interoperable, Reusable) have become essential for platform success. Standardized data formats, ontologies, and metadata schemas enable seamless integration of datasets from different sources and time periods. This standardization is crucial for training robust AI models and enabling meta-analyses across multiple programs.
Quality Control and Validation Frameworks built into platforms ensure that data quality issues are caught early and corrected systematically. Automated quality control checks, statistical process control, and anomaly detection algorithms help maintain data integrity across large-scale operations. These systems can identify systematic errors, batch effects, and outliers that might compromise downstream analyses.
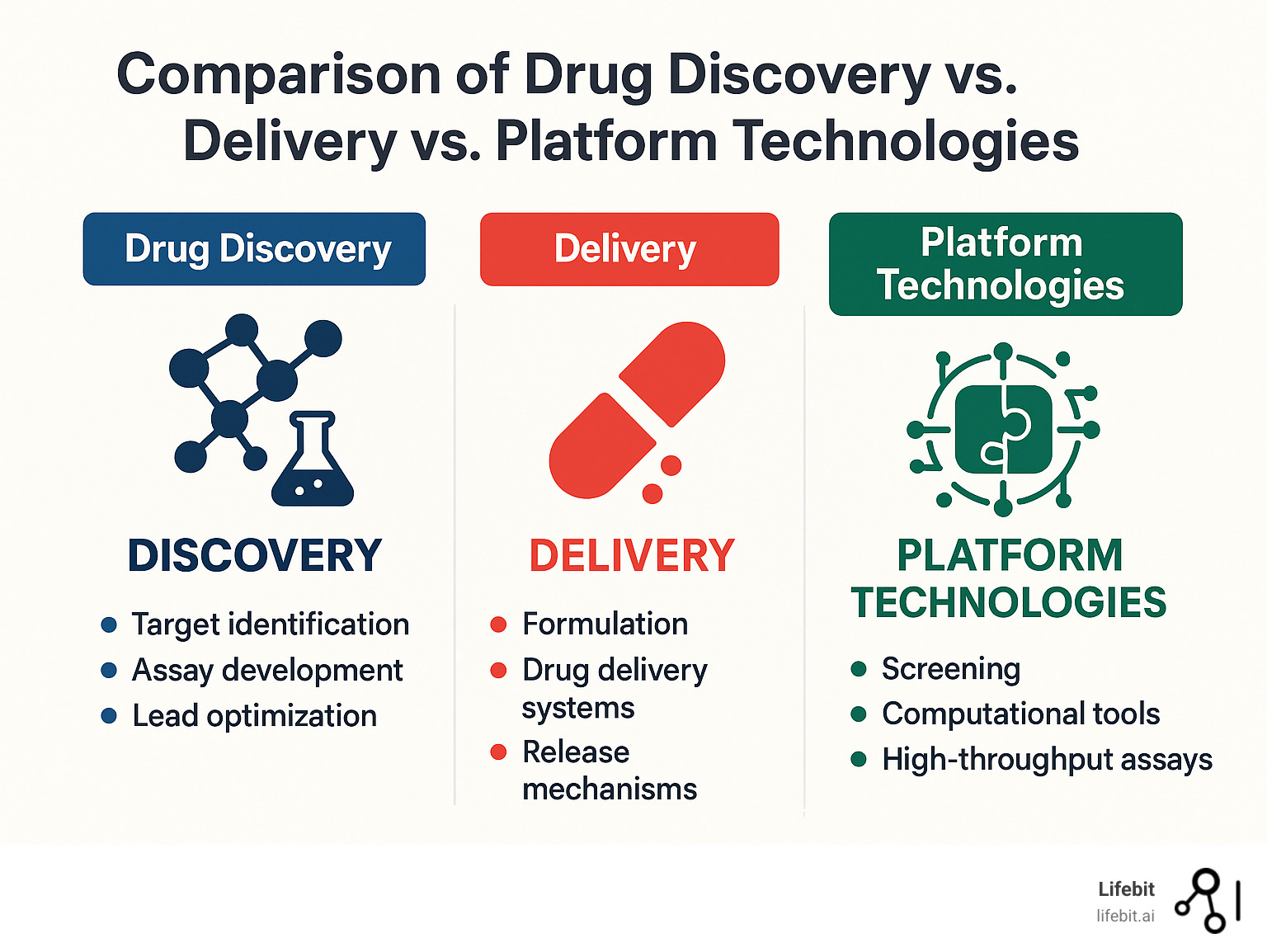
Platform Types, Technologies & Components
When we talk about a drug findy platform definition, we’re really looking at a fascinating ecosystem of interconnected technologies. The modern pharmaceutical world relies on several distinct types of platforms, each with its own specialty and unique technological requirements.
Screening platforms act like incredibly efficient matchmakers, testing thousands or even millions of chemical compounds to find the few that might become tomorrow’s medicines. These systems are remarkably productive, generating hit rates between 0.01% and 0.1%—which means finding 25-250 promising compounds from every 250,000 tested. The statistical challenge is enormous: distinguishing true biological activity from experimental noise requires sophisticated statistical methods and careful experimental design.
Computational AI platforms represent the brainy side of drug findy. They use machine learning and artificial intelligence to predict how molecules will behave, design new compounds from scratch, and optimize existing leads. This approach has become increasingly important as we face what scientists call Eroom’s Law—the ironic observation that while computing gets faster and cheaper (Moore’s Law), drug development keeps getting slower and more expensive. Modern AI platforms can process chemical libraries containing billions of virtual compounds, using deep learning models trained on millions of experimental data points.
High-throughput screening (HTS) platforms are the workhorses of the industry. These robotic systems can test 2 million molecules against a biological target in under four minutes. The miniaturization has been extraordinary: modern 1536-well plates use just 2-5 microliters per well, compared to milliliter volumes in traditional assays. This 1000-fold reduction in volume translates directly to cost savings and enables screening of precious biological targets that would be prohibitively expensive in larger formats.
In silico modeling platforms take a different approach entirely. Instead of mixing chemicals in test tubes, they use computational chemistry and quantum mechanical calculations to predict how compounds will interact with their targets. This virtual approach saves time and money by identifying the most promising candidates before expensive lab work begins. Modern platforms can perform molecular dynamics simulations on protein-drug complexes, predicting binding affinities with correlation coefficients above 0.8 compared to experimental measurements.
Nanomedicine delivery platforms focus on the final step—getting drugs to where they need to go in the body. While these are complementary to core drug findy platforms rather than part of them, they play a crucial role in turning promising compounds into effective medicines. The integration between findy and delivery platforms is becoming increasingly important as we recognize that druggability depends not just on target engagement but also on achieving therapeutic concentrations at the site of action.
The adaptability of platform approaches really shines when we look at specialized areas. Research has shown how platforms for antibiotic findy have evolved unique features to tackle antimicrobial resistance, demonstrating that platforms can be customized to address specific therapeutic challenges. These specialized platforms incorporate unique assay formats for testing compound activity against biofilms, intracellular pathogens, and resistant bacterial strains.
Screening & Phenotypic Platforms
Modern screening platforms are marvels of miniaturization and automation. High-throughput screening robots have transformed compound testing from a slow, manual process into an industrial-scale operation. Today’s most advanced systems can process 32 million wells on a single chip, using DNA barcoding on tiny beads to track millions of compounds with pinpoint accuracy.
Assay miniaturization has been a game-changer for both cost and efficiency. By shrinking experiments from traditional test tubes to 384-well or even 1536-well plates, researchers use far less expensive reagents while actually improving data quality. The physics of small volumes provides better temperature control, faster mixing, and reduced evaporation effects that can compromise larger-scale assays.
PAINS filters solve a frustrating problem that plagued early screening efforts. PAINS stands for Pan-Assay Interference Compounds—basically, molecules that give false positive results across multiple tests. These computational filters help researchers avoid chasing chemical red herrings and focus on compounds that are genuinely active. Modern PAINS filters incorporate machine learning models trained on millions of screening results to identify problematic chemical motifs with high precision.
Phenotypic readouts add another layer of insight by observing how compounds affect whole cells or even entire organisms. This approach can catch therapeutically relevant activities that purely target-focused screens might miss. High-content imaging platforms can now measure hundreds of cellular parameters simultaneously, creating rich phenotypic profiles that reveal mechanism of action and potential side effects.
3D cell culture and organoid platforms represent the cutting edge of phenotypic screening. These systems use tissue-engineered models that better recapitulate human physiology compared to traditional 2D cell cultures. Organoid platforms can model complex diseases like cancer, neurodegeneration, and infectious diseases with unprecedented fidelity, though they require specialized handling and analysis methods.
Computational & AI-Driven Platforms
The computational side of drug findy has exploded in recent years. Virtual screening capabilities now allow researchers to test billions of virtual compounds against protein targets before synthesizing a single molecule in the lab. Modern virtual screening platforms combine multiple approaches: pharmacophore modeling, molecular docking, and machine learning-based scoring functions to rank compounds by their likelihood of activity.
Deep learning models have become sophisticated enough to predict molecular properties and even generate entirely new chemical structures. Graph neural networks can learn directly from molecular structure, while transformer models adapted from natural language processing can generate novel compounds with desired properties. Scientific research on AI-designed drug candidates shows both the incredible promise and the ongoing challenges—while AI can dramatically speed up compound design, there’s still no substitute for careful experimental validation.
Digital twins and virtual patient populations represent the cutting edge of AI applications. These models can simulate how drugs might behave in different patient groups before any clinical testing begins. Virtual patient models incorporate genetic variation, disease progression models, and pharmacokinetic/pharmacodynamic relationships to predict clinical outcomes.
Hybrid quantum computing is just beginning to make waves in pharmaceutical research. Partnerships between AI companies and quantum computing platforms promise to accelerate the physics-based simulations and molecular modeling that underpin modern drug design. Quantum algorithms for molecular simulation could solve optimization problems that are intractable for classical computers.
Natural Language Processing (NLP) platforms extract insights from the vast biomedical literature, patent databases, and clinical trial records. These systems can identify novel drug-target associations, predict drug repurposing opportunities, and monitor competitive intelligence across the pharmaceutical landscape.
Drug Delivery Platforms as Complements
Here’s where we need to clear up a common confusion in the drug findy platform definition. Drug delivery platforms work alongside findy platforms, but they serve different purposes. Findy platforms find and optimize the drugs themselves, while delivery platforms figure out how to get those drugs to the right place in the body.
Nanoparticles sized around 50-200 nanometers can carry drugs directly to specific tissues. The first FDA-approved nanodrug hit the market back in 1995, and we’ve seen steady progress since then. Phase III trials for nanoparticle therapies like Abraxane® have shown better treatment outcomes compared to traditional drug formulations. Modern nanoparticle platforms can incorporate targeting ligands, pH-sensitive release mechanisms, and imaging agents for theranostic applications.
Lipid nanoparticles (LNPs) became household names during the COVID-19 pandemic, thanks to mRNA vaccines. These tiny delivery vehicles can carry both water-loving and fat-loving drugs, with total lipid doses ranging from 0.31 mg to 1.93 mg per dose depending on the specific formulation. The success of mRNA vaccines has sparked intense interest in LNP platforms for protein replacement therapies, gene editing, and cancer immunotherapy.
Liposomes and controlled release systems provide sustained drug release, maintaining optimal drug levels while reducing side effects. Modern controlled release platforms can achieve zero-order release kinetics, delivering constant drug levels for weeks or months from a single administration.
The key takeaway is that these delivery technologies complement rather than replace drug findy platforms. They’re different tools for different jobs—findy platforms identify the right medicine, while delivery platforms ensure it gets to the right place at the right time. The integration between findy and delivery is becoming increasingly important as we recognize that successful drugs must be optimized for both target engagement and pharmaceutical properties.
How Platforms Accelerate Development & Real-World Examples
When we look at how drug findy platform definition translates into real-world impact, the numbers tell a compelling story. The traditional approach to drug development often feels like watching paint dry—except the paint costs billions of dollars and takes over a decade to finish.
Cycle-time reduction happens because platforms eliminate the waiting game between different stages of development. Instead of finishing one experiment, analyzing results, planning the next step, and starting over, everything flows together. Teams report cutting early findy timelines by 30-50% when computational predictions and lab work happen simultaneously rather than sequentially.
The hit-to-lead optimization speed improvements are particularly exciting. Traditional medicinal chemistry involves a lot of educated guessing—synthesize a compound, test it, analyze what happened, then make your best guess about what to try next. Platform approaches flip this around by using AI models trained on massive datasets to predict which modifications are most likely to work before anyone steps into the lab.
Cost-saving potential varies widely, but the documented examples are impressive. Some organizations report 40% time savings for their bioinformatics teams and 395% return on investment from platform implementations. These aren’t universal guarantees—they reflect specific situations where platforms solved particular bottlenecks—but they show what’s possible when everything works together smoothly.
The most important benefit might be success-rate improvements. The pharmaceutical industry’s 90%+ failure rate has persisted for decades, partly because many decisions get made with incomplete information. Platforms can’t eliminate all uncertainty, but they provide much richer data for critical go/no-go decisions throughout development.
Adaptive trials represent another acceleration opportunity. Instead of designing a complete study upfront and hoping it works, adaptive approaches allow modifications based on accumulating data. This flexibility can save months or years when combined with platform-generated insights about patient selection and dosing strategies.
High-Impact Metrics & Benefits
The scale of modern platforms is genuinely impressive. High-throughput systems can screen 1 million compounds per day, generating datasets that would have been unimaginable just a few years ago. This isn’t just about speed—it’s about exploring chemical space more thoroughly than ever before.
The economics become particularly interesting when we consider the 22× price differential between biologics and small molecules on a daily dose basis. Platforms that can either accelerate biologic development or identify small molecule alternatives to biologic targets offer substantial value to both developers and patients.
Federated data reuse multiplies the impact of individual studies. Our work with federated genomics platforms has shown how combining datasets from multiple organizations can increase statistical power by orders of magnitude. Instead of each organization working with their own limited dataset, federated approaches open up insights that no single dataset could provide.
Prominent Platform Success Stories
Targeted oncology showcases how platforms handle complexity that would overwhelm traditional approaches. Developing targeted cancer therapies requires simultaneously optimizing the drug and identifying which patients will benefit. Platform approaches can handle drug development and companion diagnostic validation in parallel, dramatically reducing time to market.
mRNA vaccines provided the most visible recent demonstration of platform power. The speed of COVID-19 vaccine development wasn’t just about urgency—it was possible because companies had already built and validated mRNA delivery platforms. When the pandemic hit, they could focus on the specific target rather than rebuilding everything from scratch.
CRISPR therapeutics demonstrate how one platform technology can accelerate multiple programs. Once the basic gene editing platform was established and proven safe, it could be adapted to numerous genetic diseases with relatively minor modifications.
AI-first molecules are beginning to enter clinical trials, though with mixed results so far. The DSP-1181 compound designed by AI reached development four times faster than traditional approaches—but ultimately failed in Phase I trials. This highlights both the promise and current limitations of purely computational approaches.
Challenges, Regulation & Future Outlook
Building effective drug findy platform definitions sounds great in theory, but the reality is messier. Data silos are probably the biggest headache. Even within a single company, the chemistry team’s data might live in one system, the biology team’s results in another, and the clinical data somewhere else entirely.
Reproducibility issues keep scientists up at night. When an AI model makes a prediction based on proprietary data and secret algorithms, other researchers can’t easily check the work. This goes against everything science stands for, but it’s a real problem when companies need to protect their competitive advantages.
Intellectual property tangles get complicated fast. When platforms combine technologies from multiple sources, figuring out who owns what becomes like untangling Christmas lights—frustrating and time-consuming.
Interoperability standards are still catching up with the technology. Without common ways for different platforms to talk to each other, we end up with the equivalent of having an iPhone charger when everyone else uses USB-C.
The good news is that regulators are paying attention. The FDA’s Platform Technology Designation program represents a major step forward. This program lets companies reuse safety and manufacturing data across multiple products that share the same platform technology. The review process takes just 90 days, which is lightning-fast by regulatory standards.
Regulatory Landscape & Compliance
The FDA’s Critical Path Initiative has been pushing for exactly these kinds of innovative approaches to drug development. Platform technologies fit perfectly because they provide standardized, well-tested systems that reduce guesswork in regulatory submissions.
Adaptive trial designs are becoming more popular, and they need platform capabilities to handle complex protocols and analyze data in real-time. Think of it like having GPS navigation that adjusts your route based on traffic conditions, rather than printing out directions before you leave the house.
Real-world evidence requirements from regulators mean platforms need to connect clinical trial data with information from actual patient experiences. At Lifebit, our federated approach makes this possible while keeping patient data secure and private—no one wants their medical information floating around unprotected.
The European Medicines Agency has similar programs for qualifying platform technologies, though the specific requirements differ from FDA guidelines. The scientific guidance on FDA platform technology provides detailed requirements for companies seeking designation.
Evolving Science & Technology Horizon
The future of drug findy platforms looks pretty exciting, even if some of it still feels like science fiction.
Genomics integration keeps expanding as we learn more about how individual genetic differences affect drug responses. Platforms that can predict which patients will respond best to specific treatments could dramatically improve success rates.
Nanotechnology advances are creating new ways to deliver drugs exactly where they need to go. When drug findy platforms team up with nanotechnology, we might see entirely new types of medicines that work better and cause fewer side effects.
Quantum computing applications are still mostly experimental, but they show promise for solving molecular puzzles that would take regular computers forever to figure out.
Precision medicine initiatives require platforms that can juggle multiple types of data and provide personalized treatment recommendations. This is technically challenging and regulatory agencies are still figuring out how to handle it, but the potential for more effective treatments is huge.
Our Trusted Research Environment (TRE) and Trusted Data Lakehouse (TDL) models at Lifebit represent the kind of federated, secure infrastructure that the future of drug findy will require. These systems enable real-time collaboration while maintaining the strict security and compliance standards that healthcare demands.
Frequently Asked Questions about Drug Findy Platform Definition
What distinguishes a drug findy platform from a drug delivery system?
Think of it this way: a drug findy platform definition centers on finding the right medicine, while a drug delivery system focuses on getting that medicine to the right place in your body.
The scope difference is pretty straightforward. Drug findy platforms handle the detective work—identifying disease targets, screening millions of compounds, and optimizing molecules until they become promising drug candidates.
Drug delivery systems take over once you’ve already found your medicine. They’re the specialists in packaging and transport. These systems use nanoparticles, liposomes, and other clever formulation technologies to make sure your drug gets absorbed properly, reaches the right tissues, and stays active long enough to do its job.
The workflow scope tells the whole story. Findy platforms span everything from “What protein should we target?” to “Is this compound safe enough for human testing?” Delivery platforms ask different questions: “How do we get this drug past the stomach acid?” or “Can we design a nanoparticle that only releases medicine inside cancer cells?”
Integration levels also differ significantly. Findy platforms must juggle genomic data, chemical libraries, biological assays, and computational predictions all at once. Delivery platforms typically focus more narrowly on materials science and pharmaceutical formulation—still complex, but with a clearer focus.
How does artificial intelligence fit into a modern drug findy platform definition?
AI has become the brain of modern drug findy platforms. Traditional drug development was like trying to solve a billion-piece jigsaw puzzle blindfolded. AI gives us the ability to see patterns and make educated guesses about which pieces might fit together.
Predictive analytics help researchers make smarter choices about where to spend their time and money. Instead of testing every compound that looks interesting, AI models trained on billions of molecular interactions can predict which ones are most likely to work.
Generative design capabilities are perhaps the most exciting development. These AI systems can actually dream up entirely new molecules that have never existed before. They’re not just finding needles in haystacks—they’re designing better needles from scratch.
Decision support systems help researchers make sense of the massive amounts of data that modern platforms generate. When you’re screening millions of compounds and generating terabytes of data, human brains need help spotting the meaningful patterns.
Quality control applications use AI to catch problems before they become expensive mistakes. The systems can identify compounds that are likely to be false positives, flag experimental data that looks suspicious, and maintain data integrity across huge screening campaigns.
But here’s the important caveat: AI predictions are only as good as the experiments that validate them. Even the smartest AI can’t replace the need to actually test compounds in real biological systems.
Do regulatory agencies approve platforms or the drugs produced on them?
This is where things get interesting from a regulatory perspective. Traditionally, agencies like the FDA have focused on approving individual drug products rather than the platforms used to find them. But that’s starting to change in some important ways.
The FDA’s Platform Technology Designation program represents a significant shift in thinking. Under Section 506K, companies can now get formal recognition for their platform technologies. This means if you’ve built a really solid platform—whether it’s a lipid nanoparticle system or a federated AI platform like ours at Lifebit—the FDA can acknowledge that your platform itself has value.
Here’s why this matters: once your platform gets designated, you can leverage prior safety and manufacturing data across multiple products that use the same platform technology. It’s like getting a stamp of approval that says “This platform is well-characterized and reliable,” which can save months or years of duplicated testing for future products.
Product-specific review still remains the main event, though. Every single drug candidate must prove its safety and effectiveness, regardless of how sophisticated the platform that found it might be. The FDA isn’t going to approve a drug just because it came from a fancy AI platform—the compound itself still needs to work and be safe.
The key insight is that platform designation makes development more efficient without lowering safety standards. It’s about reducing redundant testing and paperwork, not about cutting corners on drug safety.
Conclusion
The journey through drug findy platform definition reveals a change that’s reshaping how we develop life-saving medicines. We’ve moved from scattered, individual experiments to integrated systems that connect every step from target identification to clinical candidates.
This shift isn’t just about technology—it’s about survival in an industry where costs have ballooned to $2.8 billion per approved drug and timelines stretch beyond a decade. The old ways simply aren’t sustainable when 90% of drug candidates fail and patients wait years for breakthrough treatments.
Future-ready platforms share several essential characteristics that set them apart from traditional approaches. They accept federated architectures that let organizations collaborate securely without compromising sensitive data or regulatory compliance. These systems integrate AI-driven analytics that can process massive multi-omic datasets and deliver insights that would take human researchers months to uncover.
Most importantly, successful platforms build regulatory compliance into their foundation rather than treating it as an afterthought. The FDA’s Platform Technology Designation program has made this approach not just smart but strategically necessary for competitive advantage.
The beauty of modern platforms lies in their modular flexibility. Like building blocks that can be reconfigured for different projects, these systems adapt to new therapeutic areas and emerging technologies without requiring complete rebuilds.
At Lifebit, we’ve witnessed this evolution while building our federated AI platform. Our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) components work together seamlessly. They provide secure access to global biomedical data while enabling the advanced analytics that modern drug findy demands.
The regulatory landscape continues evolving to support these platform approaches. When agencies like the FDA create specific pathways for platform validation and data reuse, they’re acknowledging that the future belongs to systematic, integrated approaches rather than one-off experiments.
Collaborative ecosystems represent the next frontier. The most successful drug findy programs will be those that can securely share data, generate AI-driven insights, and maintain regulatory compliance across entire pharmaceutical value chains. Organizations that accept this platform-driven future will be the ones developing tomorrow’s breakthrough therapeutics.
The drug findy platform definition we’ve explored throughout this guide isn’t just an academic concept—it’s a roadmap for more efficient, effective, and ultimately more successful therapeutic development. As these platforms mature and regulatory frameworks solidify, we’re entering an era where good science and smart technology can finally work together to bring new medicines to patients faster than ever before.
For more information about how federated data platforms can accelerate your drug findy programs, visit our platform page to learn about our comprehensive solutions for biomedical research and pharmaceutical development.


