Genomes: Your Amazing 3 Billion Blueprint
What Are Genomes and Why Do They Matter?
Genomes are the complete set of genetic instructions that make you uniquely you. Think of your genome as the ultimate instruction manual – containing all the information needed to build, maintain, and operate your body throughout your entire life.
Key facts about genomes:
- Complete DNA set: Every genome contains all genetic material in an organism
- Massive scale: The human genome has ~3.2 billion DNA building blocks (nucleotides)
- Universal presence: Every living thing has a genome, from viruses to humans
- Individual uniqueness: Your genome differs from others by about 0.1%
- Location: Found in cell nuclei (and mitochondria in humans)
The human genome project, completed in 2001, revealed that our genetic blueprint would fill 200 telephone directories if printed out, or create a stack of books 200 feet high. Even more remarkable – if you stretched out all the DNA from a single human cell, it would reach approximately six feet in length.
Understanding genomes has revolutionized medicine, enabling personalized treatments, disease prediction, and drug development. Today, what once took years and billions of dollars to sequence can be done in hours for hundreds of dollars.
As Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit with over 15 years of expertise in computational biology and genomes research, I’ve witnessed how genomic data is changing healthcare through secure, federated analysis platforms. My work spans from contributing to Nextflow – a breakthrough workflow framework used worldwide in genomic data analysis – to building cutting-edge tools that empower precision medicine across global pharmaceutical and public sector organizations.
The Blueprint of Life: Unpacking the Genome’s Structure
Your genome is nature’s instruction manual, written in a four-letter language. This manual is made of DNA (deoxyribonucleic acid), and its letters are Adenine (A), Thymine (T), Cytosine (C), and Guanine (G).
These letters follow strict pairing rules: A always pairs with T, and C always pairs with G. Millions of these pairs stack up to form the rungs of a twisted ladder—the famous double helix structure finded by James Watson and Francis Crick.
Amazingly, the DNA from one of your cells would stretch about six feet in length. This enormous molecule fits inside a microscopic cell nucleus because it wraps around proteins called histones, like thread on a spool. This creates compact bundles called chromosomes. Humans have 23 pairs of chromosomes (46 total) in most cells.
Scattered along these chromosomes are genes—specific sections that contain instructions for making the proteins and functional RNA molecules that keep your body running smoothly.
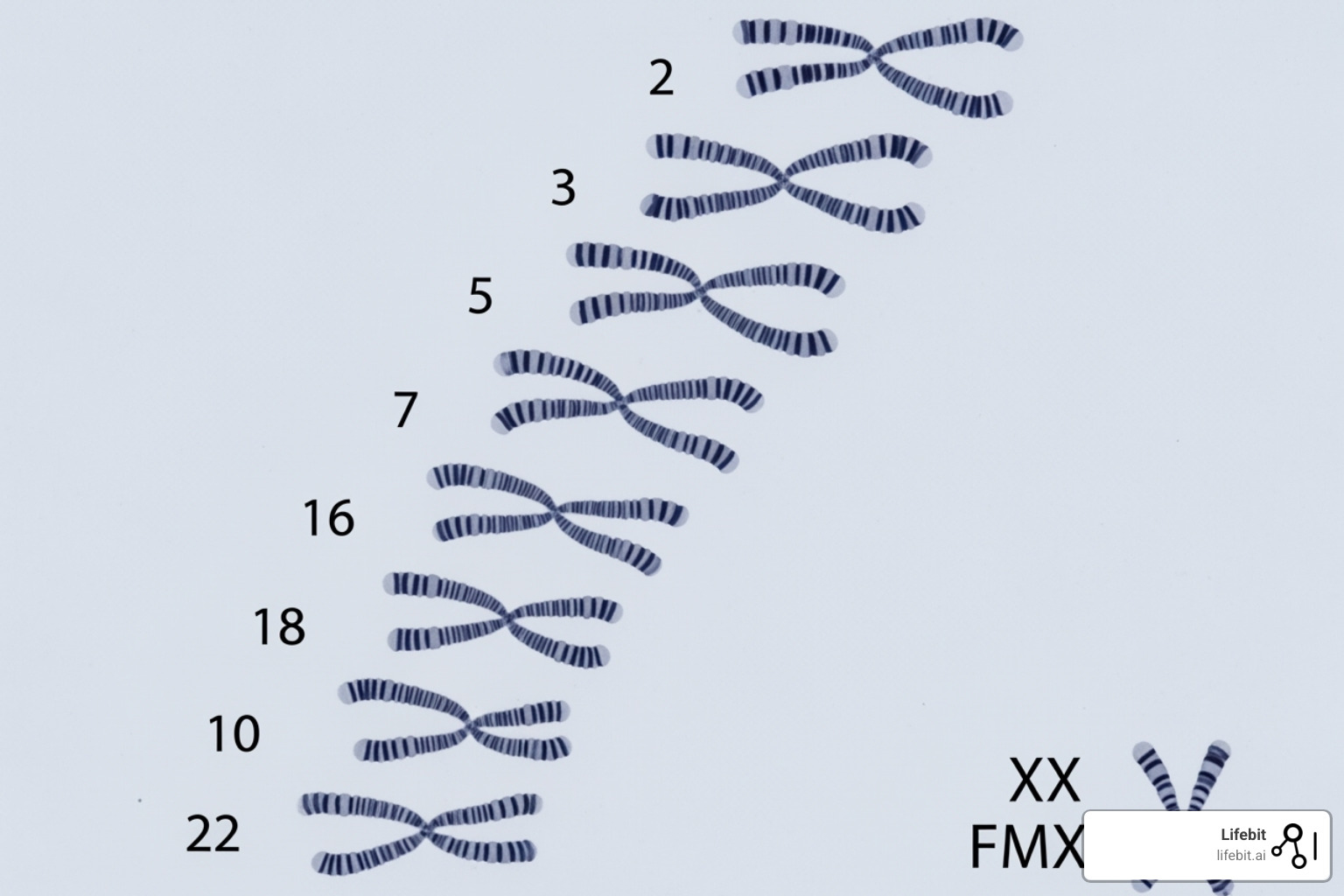
Where is the genome located?
The location of your genome depends on the cell type.
In eukaryotic cells (like yours), most of your genome lives in the nucleus, organized into linear chromosomes. However, a second, much smaller genome exists in your mitochondria.
Mitochondrial DNA is tiny compared to your nuclear genome—only about 16,000 base pairs. This circular DNA comes almost entirely from your mother, making it useful for tracing maternal lineages.
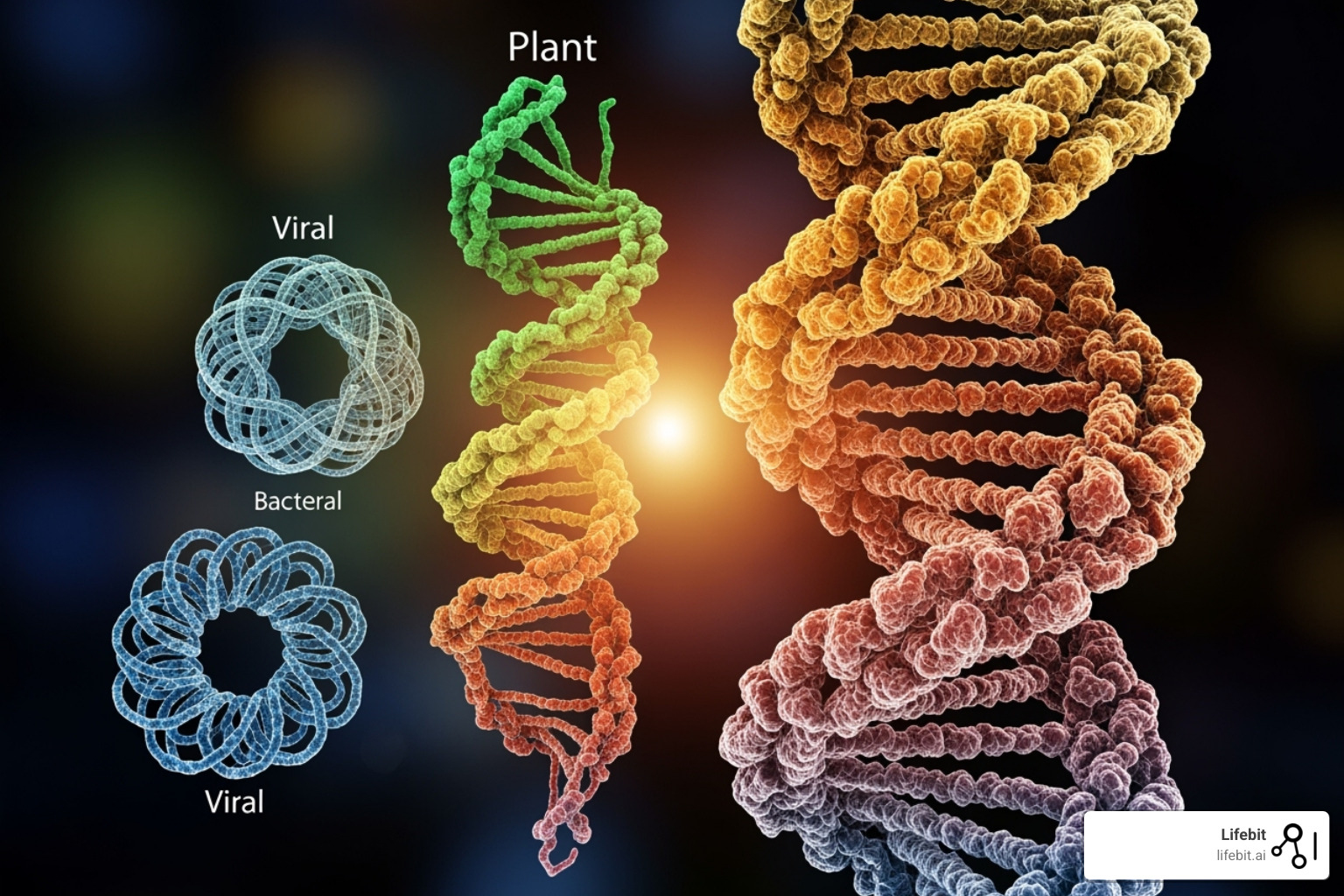
Prokaryotic cells like bacteria lack a nucleus, so their genome—usually one circular DNA molecule—floats in a region called the nucleoid. Many also carry extra DNA circles called plasmids.
Viruses are the wild cards. Their genetic material can be DNA or RNA, single- or double-stranded, and linear or circular. The first genome ever sequenced (1977) belonged to the virus φX174, with just 5,386 base pairs.
Coding vs. Non-Coding: The Building Blocks of Genomes
Surprisingly, only about 1-2% of your entire genome actually codes for proteins. The other 98-99% has different functions.
The protein-coding parts are called exons. Your genome also contains introns—sequences that are transcribed but then edited out before the final protein is made.
For decades, scientists called much of this non-coding region “junk DNA.” We now know this DNA is incredibly important, containing regulatory sequences that act like genetic switches to control when and where genes are active.
Your genome is also packed with repetitive elements. More than 45% of your genome consists of transposable elements, or “jumping genes,” which can move around your genome.
One successful example is the Alu element. This sequence, about 350 base pairs long, makes up roughly 11% of the human genome, with around 1.5 million copies.
The story of non-coding DNA is still being written. Scientific research on non-coding RNA continues to reveal new functions for these mysterious parts of our genomes, proving that nature rarely keeps anything truly useless around.
Decoding the Code: How We Study and Sequence Genomes
Understanding genomes requires reading the genetic code through a process called genome sequencing. The journey begins with DNA extraction, where scientists carefully separate genetic material from cells.
The story of sequencing technology is fascinating. In the mid-1970s, Fred Sanger developed the first methods to read DNA sequences. Sanger sequencing was for its time but was also slow and expensive.
Everything changed with Next-Generation Sequencing (NGS) in the early 2000s. Instead of reading one DNA fragment at a time, NGS platforms can simultaneously sequence millions or even billions of fragments. This leap is due to massively parallel sequencing, where millions of DNA fragments are sequenced simultaneously. Major platforms like Illumina use a ‘sequencing-by-synthesis’ approach. Newer ‘third-generation’ technologies from Pacific Biosciences (PacBio) and Oxford Nanopore read much longer single DNA molecules, crucial for assembling complex genomes. Modern labs can now sequence over 100,000 billion bases per year.
Generating sequence data is only half the battle. Bioinformatics uses powerful computers and algorithms to piece together millions of short DNA fragments into complete genomes. This process, called genome assembly, is like assembling a jigsaw puzzle with billions of pieces and requires enormous processing power. The primary challenge in assembly is handling repetitive sequences. Short reads from these regions are identical, making it hard for algorithms to place them correctly. Bioinformaticians overcome this by combining short-read and long-read data to bridge these gaps and create a more complete genomic picture.

The Human Genome Project: A Landmark Achievement
The Human Genome Project (HGP), launched in October 1990, was a monumental undertaking. The scale was staggering: sequencing 3.2 billion letters, where a single typo could mean the difference between health and disease.
Scientists from around the world joined forces in an unprecedented collaboration. The project’s ambitious goals included mapping all 23 pairs of human chromosomes, developing better sequencing technologies, identifying genes, and addressing the resulting ethical questions.
The first draft of the human genome was announced in February 2001, years ahead of schedule. The project officially wrapped up in 2003, and its impact continues to ripple through science and medicine. The cost reduction has been dramatic: what once required billions of dollars now costs hundreds.
However, the HGP couldn’t crack every genetic puzzle. It wasn’t until April 2022 that the Telomere-to-Telomere (T2T) Consortium filled in the missing pieces, delivering the first truly complete human genome sequence.
Key Milestones in Genome Sequencing
The path to reading genomes is marked by several key achievements:
| Year | Organism | Historic Significance |
|---|---|---|
| 1977 | Virus φX174 | First genome ever sequenced – a tiny DNA virus with just 5,386 letters |
| 1995 | Haemophilus influenzae | First bacterial genome completed, proving large genomes could be sequenced |
| 1996 | Baker’s yeast | First complex cell genome – a major leap in complexity |
| 2001 | Human (draft) | The famous first draft that changed everything |
| 2022 | Human (complete) | Every single gap finally filled by the T2T Consortium |
Each milestone pushed scientific boundaries, revealing secrets about how life works and laying the foundation for today’s genomic medicine, where understanding genomes helps doctors provide personalized treatments and predict diseases.
A World of Difference: The Diversity of Genomes
You might think complex organisms have bigger genomes, but nature is full of surprises. This is known as the C-value paradox, a fascinating mystery in genomics.
Consider this: humans have a genome with about 3.2 billion nucleotides. Yet the Japanese flower Paris japonica has a genome containing roughly 150 billion nucleotides—that’s 50 times larger than ours! Other examples abound: the marbled lungfish has a genome over 40 times larger than ours, and some amoebas have genomes estimated to be hundreds of times larger. This clearly demonstrates that organismal complexity is not a direct product of genome size.
This lack of correlation between genome size and complexity is largely due to the expansion and contraction of repetitive DNA elements, like the “jumping genes” mentioned earlier. On the flip side, some organisms take a minimalist approach. Genome reduction is common in symbiotic bacteria that discard genes they no longer need due to their dependence on a host.
This incredible spectrum of genome sizes shows that evolution doesn’t follow a simple “bigger is better” rule, revealing the diverse strategies life has developed to thrive.
Comparing Genomes: From Humans to Horses
Comparative genomics compares genomes across species to uncover stories about evolution. Our closest living relatives, chimpanzees, share a remarkable 98.8% similarity with human DNA. That tiny 1.2% difference accounts for all the biological and cognitive distinctions between us.
Even more distant relatives show surprising similarities. The horse genome has 32 pairs of chromosomes and 2.7 billion base pairs, while humans have 23 pairs of chromosomes and 3.2 billion base pairs. Despite these differences, both genomes contain many similar genes, reflecting a shared mammalian ancestry.
These comparisons help scientists identify essential genes, understand gene function, identify disease-causing mutations, and trace the evolutionary paths that led to today’s incredible biodiversity.
When the Code Changes: Genomic Alterations
Your genome is more dynamic than you might think. These genomic alterations, or mutations, play crucial roles in evolution and health.
Somatic mutations happen in body cells and accumulate with age. They are not inherited but can contribute to aging and diseases like cancer.
Germline mutations occur in reproductive cells (sperm or eggs) and can be inherited by children, appearing in every cell of their bodies.
The most common genetic variations are Single Nucleotide Polymorphisms (SNPs)—single-letter changes in the DNA code that help explain our individual differences.
Larger changes also occur. Insertions add extra DNA, while deletions remove it. Beyond simple insertions and deletions, genomes can undergo larger structural variations. These include duplications (a segment is copied), inversions (a segment is flipped), and translocations (a piece of one chromosome attaches to another). While often benign, these changes are linked to developmental disorders and are hallmarks of many cancers, as they can disrupt critical genes. The consequences range from harmless to beneficial to harmful. Scientific research on somatic mutations has revealed their critical role in cancer, while the expansion of a repetitive DNA sequence causes Huntington’s disease.
Understanding these genomic alterations is essential for medicine and is the raw material for evolution.
The Future of Genomics: From Research to Reality
The world of genomics is no longer confined to research laboratories. Today, insights from studying genomes are changing how we approach healthcare, making science fiction a reality for millions.
Genomic medicine is perhaps the most exciting frontier. By analyzing your unique genome, doctors can understand your health risks before symptoms appear. This genetic insight powers personalized medicine, where treatments are custom to you. For example, identifying mutations in the BRCA1 and BRCA2 genes allows for proactive screening for hereditary breast and ovarian cancer. In oncology, sequencing a tumor’s genome can reveal targetable mutations, such as in the EGFR gene in lung cancer, enabling precision treatment.
Pharmacogenomics studies how your genes influence your response to medications. These genetic variations influence how people metabolize drugs, affecting the difference between effective treatment and dangerous side effects. A classic example is the anticoagulant warfarin. Genetic testing for variations in the CYP2C9 and VKORC1 genes helps clinicians predict the optimal dose, reducing the risk of dangerous side effects. By understanding these variations, doctors can prescribe the right drug at the right dose from the start.
Gene editing tools like CRISPR act as molecular scissors, allowing scientists to make targeted changes to DNA sequences to potentially correct genetic defects. The CRISPR-Cas9 system uses a guide RNA to direct the Cas9 enzyme to a precise DNA location to make a cut. The cell’s repair mechanisms can then disable a gene or insert a new sequence. Its use in clinical trials to treat sickle cell disease marks a major milestone.
With these advances come significant responsibilities. The field of genomics grapples with important Ethical, Legal, and Social Implications (ELSI). Questions about data privacy, genetic discrimination, and equitable access loom large. To combat discrimination, the U.S. enacted the Genetic Information Nondiscrimination Act (GINA) to protect individuals from misuse by insurers and employers. Ensuring equitable access is also critical, as most genomic data comes from people of European ancestry, limiting its utility for other populations. Efforts are underway to diversify these databases.
Protecting genomic data while enabling research requires sophisticated platforms that can maintain security and compliance while facilitating collaboration across institutions and borders.
Exploring the Different Fields of Genomics
The field of genomics has branched into numerous specialized areas, each tackling different pieces of the genetic puzzle.
Functional genomics asks: what do genes actually do? This field studies how genes interact, when they’re turned on or off, and how these processes create everything from eye color to disease resistance.
Structural genomics focuses on the three-dimensional shapes of proteins encoded by our genomes. Since a protein’s shape determines its function, understanding these structures is crucial for designing new drugs.
Epigenomics explores how genes can be turned on or off without changing the underlying DNA sequence. These epigenetic modifications can be influenced by diet and stress, explaining how identical twins can develop different diseases despite sharing the same genome.
Metagenomics studies entire communities of microbes. Scientists can sequence all the genetic material in a sample—from soil, ocean water, or your gut microbiome—revealing incredible microbial diversity.
Pangenomics recognizes that not all members of a species share the same genes. This is especially important for understanding bacteria, where different strains might have different capabilities.
These diverse fields work together to build our understanding of how genomes shape life. More info about genomic medicine shows how this research translates into better treatments and healthier lives.
Frequently Asked Questions about Genomes
Understanding genomes can feel overwhelming, but many people share similar questions. Let’s explore some of the most common ones.
What is the difference between a gene and a genome?
Think of your genome as a massive cookbook for your entire body. A single gene is just one recipe in that book—for instance, the instructions for a protein that determines eye color.
Genes are the individual functional units within the much larger genome. While a gene might contain instructions for one particular molecule, your genome contains all 20,000-25,000 human genes plus vast amounts of regulatory DNA that controls them.
How much of the human genome is identical between people?
Any two humans share about 99.9% identical DNA. Despite all our obvious differences, we’re remarkably similar at the genetic level.
That tiny 0.1% difference is where all human diversity lives. These variations, called Single Nucleotide Polymorphisms (SNPs), are scattered throughout our genomes. Some influence physical traits, while others affect disease susceptibility or have no noticeable effect.
That 0.1% of 3 billion nucleotides still equals about 3 million differences between any two people, which is the basis for human diversity.
Can your genome change over time?
While the core genetic blueprint you inherit is stable, your genome is not completely static.
Somatic mutations accumulate as you age. These are changes in your body’s cells (not reproductive cells) from environmental exposures or copying errors when cells divide. Most are harmless, but some can contribute to cancer or other age-related diseases.
Environmental factors also leave their mark through epigenetic changes. These modifications act like volume controls on genes, turning them up or down based on factors like diet and stress, without altering the DNA sequence itself. These changes can even be influenced by your lifestyle and passed down to your children.
Additionally, mobile genetic elements can occasionally “jump” to new locations in your genome, potentially altering how nearby genes function. These dynamic changes mean that genomics is far more fluid than we once believed.
Conclusion
As we’ve seen, genomes are more than instruction manuals. They represent the essence of life, from the DNA double helix and complex chromosomes to the mysterious non-coding regions once called “junk.”
The progress in genome sequencing is remarkable. What once took 13 years and billions of dollars to sequence the first human genome now takes hours and costs a few hundred dollars. This shift has opened doors to an era of personalized medicine, where your unique genetic makeup guides your treatment.
Gene editing technologies like CRISPR are moving from science fiction to reality, offering hope for curing genetic diseases. Pharmacogenomics is helping doctors prescribe the right medication at the right dose for each patient.
With great power comes great responsibility. As we open up the secrets of our genomes, we must carefully steer the ethical landscape. Questions about data privacy, genetic discrimination, and equitable access to these technologies are more important than ever.
The massive amounts of genomic data being generated need sophisticated platforms that can handle the sheer volume and the security and compliance requirements of such sensitive information.
At Lifebit, we’re tackling these challenges head-on. Our federated AI platform enables researchers and healthcare organizations to collaborate securely across the globe, breaking down data silos while maintaining the highest standards of privacy and compliance. Through our Trusted Research Environment, Trusted Data Lakehouse, and R.E.A.L. platform, we’re making it possible for scientists to work with genomic data in ways that were previously impossible.
The future of genomics depends on creating an infrastructure that allows science to flourish safely and ethically. Every breakthrough brings us closer to a world where genetic diseases become treatable, medications work better, and precision medicine becomes the standard of care.
Learn how Lifebit’s federated platform powers large-scale research and find how we’re helping to shape a healthier future, one genome at a time.

