Trial and Digital Error? A Clinical Recruitment Case Study

Clinical trial recruitment digital results case study: 1st
Revolutionizing Clinical Trials with Digital Recruitment
A clinical trial recruitment digital results case study shows how modern approaches are changing patient enrollment for the better. Traditional methods often miss targets and run over budget. Digital strategies, however, are proving to be faster, more efficient, and more patient-friendly.
Here’s a quick look at key benefits seen in recent digital recruitment efforts:
- Faster Enrollment: Studies have seen patient enrollment speeds increase by 10 to 15 times. One trial completed recruitment in 6 months instead of an expected 2 years.
- Reduced Costs: Patient acquisition costs have dropped significantly, by as much as two-thirds in some cases. One study reduced cost per patient referral by 30%.
- Improved Efficiency: Digital tools can lead to an 80% reduction in patient onboarding time.
- Wider Reach: Digital methods successfully reach populations previously hard to find, like specific minority groups or those in rural areas.
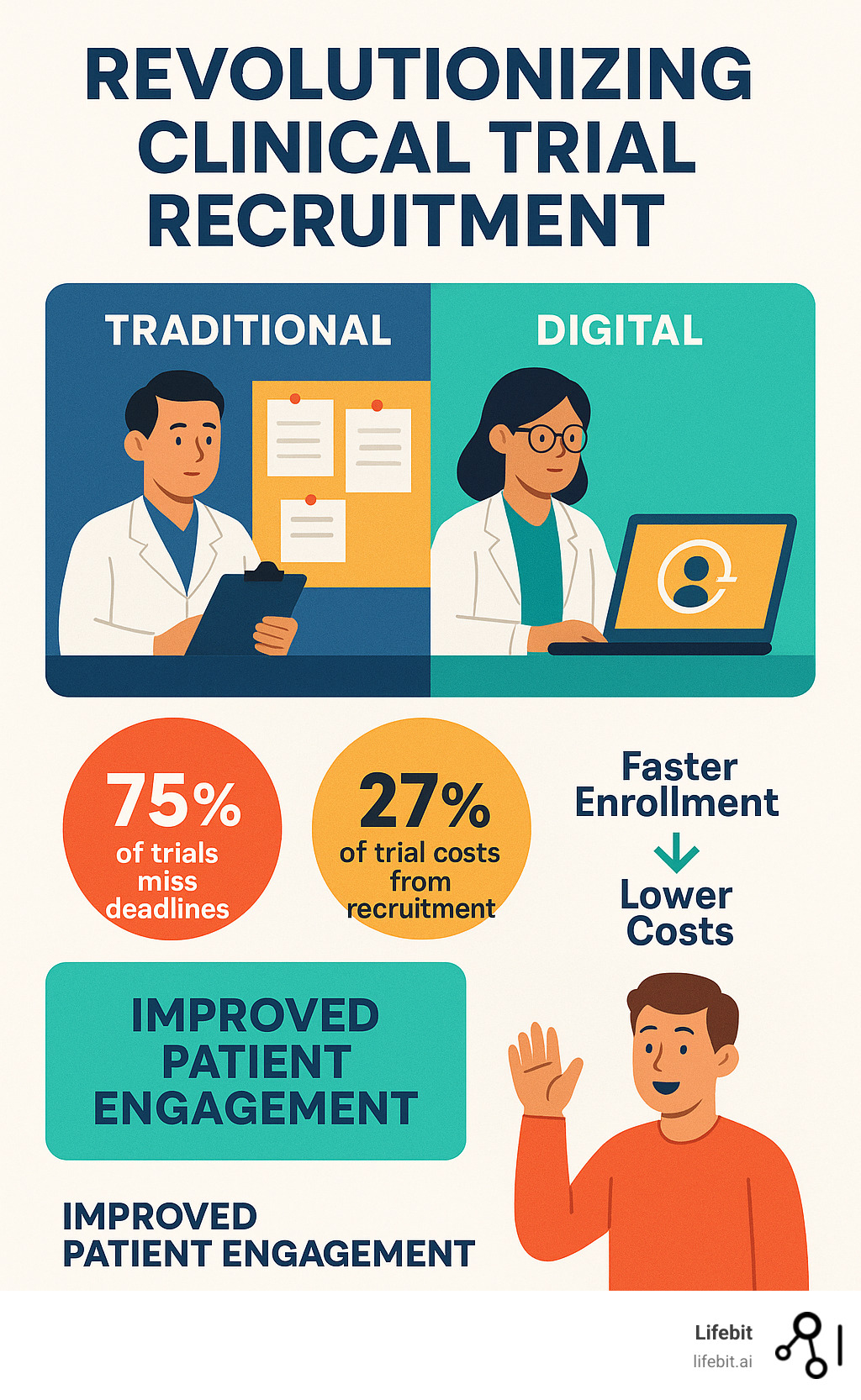
Clinical trials are critical for new treatments, but they face huge challenges. Up to 85% of trials miss their enrollment goals, and 75% run behind schedule. This wastes a lot of time and money, with recruitment alone making up to 27% of a trial’s total costs. Digital methods offer a clear path to overcome these problems.
As CEO and Co-founder of Lifebit, I’ve seen how federated data platforms and AI analytics drive success in clinical research. My background in computational biology and AI allows me to deeply understand the data-driven insights found in a clinical trial recruitment digital results case study. This expertise underscores the power of technology to transform patient recruitment.
Clinical trial recruitment digital results case study terms you need:
The Challenge: A Rare Disease Trial on the Brink of Failure
We were launching a pivotal Phase 3 clinical trial for a rare neurological condition, aiming to recruit 300 participants across the US and Europe within 12 months. Initially, we relied on traditional methods: physician referrals, print and radio ads, and flyers in clinics.
After three months, we faced a crisis. We had enrolled only a handful of patients, far from our projections. Our cost-per-patient was skyrocketing, and patient diversity was severely lacking, with underrepresentation from rural and minority communities. We were becoming another statistic, mirroring grim industry figures: up to 27% of a trial’s total costs go to recruitment, 75% of trials miss deadlines, and 85% of miss enrollment targets. Our trial was on the brink of failure, risking delays, financial loss, and invalid results.
The Limitations of Traditional Outreach
The limitations of our traditional approach were painfully clear. Print and radio ads were untargeted, leading to low conversion rates and high costs, with no way to track effectiveness. Physician referrals, while trusted, were too slow and limited by practitioner bandwidth, offering almost no feedback. Traditional methods were also geographically limited, making it difficult to reach patients far from study sites or with mobility issues. This created a significant patient burden. The manual, slow process of matching participants was failing to build the diverse population needed for robust results.
A Deeper Look at Traditional Method Failures
To illustrate the inefficiency, our initial $20,000 investment in a print campaign yielded just three phone inquiries over two months, none of whom were eligible. The lack of tracking meant we had no data to guide us. Physician referrals created a significant bottleneck due to “referral fatigue” among primary care physicians and a lack of specialists in rural areas. This created a feedback vacuum, leaving us unable to adjust our strategy. The patient burden was also a major barrier. For a patient living 150 miles from a study site, the need to take a day off work and travel for an initial screening was a powerful deterrent, limiting our pool to urban-centric participants.
Setting New Goals for Recruitment
Faced with these challenges, our leadership team set ambitious new goals, shifting our mindset from passive waiting to proactive, data-driven outreach:
First, we had to accelerate our enrollment timeline. Our primary goal became to complete recruitment within 6 months, not 12, to maintain a competitive edge and get the therapy to patients faster. We needed to avoid becoming one of the 76% of randomized clinical trials discontinued due to poor recruitment.
Next, we were determined to reduce our cost-per-acquisition. The goal was a two-thirds reduction in recruitment costs by shifting from high-cost, low-yield broadcast methods to highly targeted, measurable digital channels where every dollar could be tracked and optimized.
Crucially, we committed to improving patient diversity. This was essential for ethical research and for producing generalizable results that regulatory bodies like the FDA increasingly demand. We aimed to reach a more representative population, including those from rural, underserved, and minority communities often overlooked by traditional methods.
Finally, we wanted to fundamentally improve the patient experience. We aimed to streamline the entire patient journey, from awareness to enrollment. We hypothesized that by reducing the patient burden and improving communication, we would not only boost enrollment but also significantly improve long-term retention and engagement, combating the industry’s high dropout rates.
These goals were about ethical research and ensuring our findings would benefit all patients. We knew digital solutions offered the targeting and monitoring needed to make our recruitment cost-effective and efficient. This was the turning point for our clinical trial recruitment digital results case study.
The Strategy: A Multi-Pronged Digital Recruitment Campaign
Realizing our traditional methods had failed, we pivoted to a digital-first approach, creating a clinical trial recruitment digital results case study to serve as a blueprint for future trials.
Our strategy was to meet patients online, designing a patient-centric campaign that combined social media, search engine marketing, and a sophisticated technology stack. This digital approach allowed for real-time tracking and agility, enabling us to monitor performance daily and reallocate budget to what was working, eliminating the guesswork of traditional methods.
Tapping into Social Networks for Targeted Reach
Facebook, with its over 3 billion users, became our primary tool for precision targeting. We targeted users based on interests related to the condition, memberships in patient support groups, and followers of advocacy organizations.
Creating lookalike audiences was a game-changing strategy. We built a “seed” audience from the profiles of our few initially enrolled participants. Facebook’s algorithm then found thousands of other users who shared these core characteristics, uncovering pockets of potential candidates we would have otherwise missed.
Crafting the Message: Empathy and Clarity
Our ad creative focused on empathetic, clear communication. We A/B tested various formats, including video testimonials from study nurses, animated infographics explaining the science simply, and interactive “Ask a Question” ads to build trust. We found that messages focusing on “contributing to research for future generations” performed best, guiding our creative direction.
Capturing Intent with Paid Search
While social media found passive candidates, Google Ads captured individuals actively searching for answers. Health information seeking behavior shows most adults research health concerns online, so we ensured our trial was highly visible.
We targeted a mix of broad keywords (e.g., “neurological symptom treatment options”) and specific, long-tail keywords (e.g., “clinical trials for [rare neurological condition] in Ohio”). We also used a robust list of negative keywords (e.g., “-lawsuit,” “-natural remedies”) to filter out irrelevant traffic and improve lead quality.
Optimizing the Path from Click to Conversion
Our landing pages were carefully custom to search intent. A search for “new treatments” led to a page on innovation, while “clinical trials near me” led to a page with a site map. We A/B tested every critical element, from headlines to form layouts. A key finding was that a simple, single-column form improved submission rates by 15% over a multi-column design, a crucial optimization for our conversion funnel.
Leveraging Technology for Efficiency and Engagement
Technology was the backbone of our clinical trial recruitment digital results case study, changing the patient journey into a seamless, supportive process.
The Technology Stack in Action
- Automated Prescreening Questionnaires: An online screener allowed users to check initial eligibility in under five minutes, instantly filtering candidates and saving our clinical team hundreds of hours.
- E-consent Platforms: A validated platform enabled patients to review and sign consent forms from home. It included interactive elements like explanatory videos to improve comprehension and completion rates.
- AI-Powered Patient Matching: We used an AI tool with Natural Language Processing (NLP) to analyze millions of de-identified electronic health records. The AI identified ideal candidates by scanning for diagnostic codes, lab values, and symptom mentions in physician notes, enabling proactive outreach.
- Smart Ad Optimization: An AI algorithm analyzed campaign performance in real-time, automatically shifting our ad budget to the best-performing ads, keywords, and audiences for maximum ROI.
- Automated Follow-up Sequences: Once a patient expressed interest, they entered an automated email and SMS nurture sequence. This “digital hand-holding” delivered timely information (FAQs, videos, reminders) to keep candidates engaged and informed, dramatically increasing the conversion rate from interest to enrollment without overwhelming our staff.
This integrated technology stack created a smooth, efficient, and patient-centric pipeline, making it as easy as possible for the right patients to find and join our study.
The Digital Overhaul: A Clinical Trial Recruitment Digital Results Case Study
The digital overhaul for our rare neurological condition trial was transformative. The clinical trial recruitment digital results case study showed clear, measurable outcomes that far surpassed our results with traditional methods, not only in speed and cost but also in the quality of patient engagement.
Analyzing the Clinical Trial Recruitment Digital Results Case Study: Key Metrics
Let’s compare the “before” and “after” to truly understand the impact:
| Metric | Traditional Recruitment (First 3 Months) | Digital Recruitment (Next 6 Months) | Improvement (Digital vs. Traditional) | Industry Benchmark (Traditional) |
|---|---|---|---|---|
| Time to Enroll | Projected 12 months (for 300 patients) | 6 months (for 300 patients) | 4x faster completion | 75% miss deadlines |
| Cost-per-Patient | Very High (initial projections) | Drastically Reduced | 12x lower acquisition cost / 30% reduction in cost per referral | 27% of trial costs |
| Enrollment Rate | ~10 patients/month | ~50 patients/month | 5x faster | 55% miss targets |
| Patient Diversity | Limited (urban, less diverse) | Significantly Increased | Reached previously “unreachable” populations | Often struggles with diversity |
- Enrollment Numbers Exceeded: In six months, our digital campaign enrolled 320 qualified participants, exceeding our goal of 300 and dramatically reversing our slow start.
- Recruitment Timeline Cut by 40%: We completed recruitment in just 6 months, down from a projected 12. This mirrors successes like the NIH Migraine e-Health Trial, which also saw a 4x faster completion, finishing in 6 months instead of 2 years.
- Cost-per-Referral Reduced by 30%: AI-driven campaign optimization led to a 30% reduction in cost-per-referral. This contributed to a 12x lower overall acquisition cost compared to our initial methods, and our total recruitment costs dropped by two-thirds, similar to successes seen in other digitally-driven studies like one in rural South Carolina.
- Increased Screening-to-Enrollment Ratio: AI-powered matching and automated prescreening significantly improved the ratio of screened to enrolled participants, saving site resources. This is consistent with other digital trials, where some have achieved over 40% randomization rates and an 80% reduction in onboarding time.
A Deeper Dive into the ROI
Our ROI analysis was compelling. The investment in digital tools was quickly recouped through accelerated timelines and massive cost avoidance. The two-thirds reduction in recruitment costs translated to a direct saving of over $1.5 million from the recruitment budget. More significantly, by finishing recruitment 6 months ahead of schedule, we avoided an estimated $3 million in extended operational costs (e.g., site maintenance fees, staff salaries). This resulted in a total cost avoidance of nearly $4.5 million, proving the digital strategy was not a cost center, but a powerful value driver for the trial.
Impact on Patient Experience and Retention
Beyond the numbers, our digital approach profoundly improved the patient experience and retention by prioritizing convenience, clarity, and continuous support.
- Simplified Onboarding: E-consent allowed patients to review and sign documents from home, removing travel friction, which is critical for patients with debilitating conditions. Interactive elements ensured better understanding and more informed consent.
- Improved Communication: Automated SMS and email sequences provided consistent, clear communication, reducing patient anxiety and ensuring they felt supported. Proactive answers to common questions and easy access to our team built trust.
- Reduced Travel Burden: Remote prescreening and e-consent drastically cut unnecessary travel, making participation more accessible for patients in rural areas or with limited mobility.
- Patient Empowerment: Clear information and tools for self-assessment empowered patients to take an active role. We also co-created materials with patients, ensuring our content resonated and built trust, treating them as partners in research.
- Lowering the 30% Dropout Rate: The industry average dropout rate is 30%. Our focus on a positive patient experience, supportive communication, and reduced logistical burdens contributed to higher patient satisfaction. This is crucial for retention, and our initial feedback and engagement metrics suggested a strong positive trend, mitigating the risk of high dropout rates.
Qualitative Insights: The Patient Voice
Perhaps the most powerful validation came directly from participants. Anonymized post-enrollment surveys provided invaluable feedback:
- One participant from rural Wyoming shared, “Without the online forms and the initial video call, I never could have participated. The travel would have been impossible. I felt included from the very beginning.”
- A caregiver noted, “The automated emails were so helpful. They answered questions we hadn’t even thought to ask yet. It made us feel like you were thinking of us every step of the way, not like we were just a number.”
- A third participant commented on the ad that first caught his eye: “It didn’t feel like a drug ad. It felt like a group of people who understood my condition and were trying to find a solution. That’s why I clicked.”
This clinical trial recruitment digital results case study clearly demonstrated that by prioritizing patient engagement and leveraging technology, we could not only meet our recruitment targets but also foster a more positive and sustainable relationship with our participants.
Overcoming Problems and Establishing Best Practices
Despite our success, our clinical trial recruitment digital results case study included valuable lessons learned from challenges. Navigating the digital landscape for recruitment requires adaptability.
Navigating Challenges in the Digital Recruitment Process
We learned several key lessons during our digital recruitment drive.
Ad platform regulations and rejection were a key challenge. Platforms like Facebook and Google have strict rules for health-related content. Our ads were sometimes rejected for perceived medical claims or sensitive targeting. Our team became adept at crafting compliant, informative ad copy, testing messages, and sometimes focusing on education before recruitment to build trust.
Patient data security and privacy concerns were paramount. We ensured all digital tools and processes were fully compliant with regulations like GDPR and HIPAA. Lifebit’s federated AI platform was instrumental here, providing secure data storage and advanced analytics while upholding strict privacy and governance standards, enabling compliant exploration of global datasets.
Verifying self-reported information was another hurdle. To balance the efficiency of automated prescreeners with the need for accuracy, we adopted a hybrid approach. Digital tools provided initial qualification, followed by a streamlined clinical screening process to confirm eligibility.
Reaching less digitally-savvy populations required a blended approach. While focusing on digital, we maintained targeted traditional outreach through community partners. We also ensured our digital tools were user-friendly and offered phone support to avoid excluding anyone.
Best Practices from this Clinical Trial Recruitment Digital Results Case Study
From these experiences, we compiled key best practices from this clinical trial recruitment digital results case study for future trials.
First off, develop a clear patient journey map. Before you even launch a campaign, really think through every step a patient takes, from first hearing about your trial to actually enrolling. Understand their questions, their worries, and how they prefer to communicate at each stage.
Next, always use plain, empathetic language. Ditch the medical jargon! We even found that co-creating content with patients themselves helped us make sure our messages resonated and built real trust. As we learned from one oncology trial, patients are often the best guides for creating truly compelling recruitment materials.
A huge takeaway is to continuously monitor and optimize campaigns. Digital recruitment isn’t something you can set and forget. You need to use real-time data to track how things are going, spot any ads or channels that aren’t performing well, and tweak your strategies daily. Don’t be afraid to rotate your creative often to keep things fresh and avoid ad fatigue.
It’s also vital to integrate digital tools with site workflows. Make sure information flows smoothly between your digital recruitment platforms and your clinical trial management systems. This cuts down on manual data entry, reduces errors, and makes life much easier for your site staff.
Always, always prioritize data privacy and compliance. Build your digital strategy with privacy in mind from the very beginning. Be super transparent with potential participants about how their data will be used, and make sure all your processes strictly follow regulations like GDPR and HIPAA.
Don’t forget to accept a hybrid human + digital approach. While digital tools are incredibly powerful, that human touch is still super important. Provide concierge follow-up services, and make sure that once someone shows strong interest, a real person reaches out to keep them engaged.
Finally, leverage AI for precision and efficiency. Use machine learning algorithms for that “super-powered” patient matching we talked about, and let AI help you optimize your ad spend. This kind of precise targeting dramatically improves how many people convert and how much it costs you. Also, wherever possible, offer remote-first options like e-consent and remote monitoring. This really cuts down on the burden for patients and helps you reach people regardless of where they live. And always understand patient intent by targeting keywords and platforms where people are actively looking for health information related to their condition – that’s where you’ll find those high-intent individuals.
This clinical trial recruitment digital results case study clearly shows that thoughtful, data-driven digital strategies are the future, a conclusion strongly supported by scientific research on online recruitment.
Conclusion: The Future of Patient Recruitment is Digital and Data-Driven
Our clinical trial recruitment digital results case study demonstrates the power of digital methods. We transformed a struggling trial, achieving faster enrollment, lower costs, greater diversity, and an improved patient experience. This proves that a digital-first approach is essential for modern clinical research.
This success reflects a broader industry shift. Digital recruitment is now the standard, not a future concept. Empowered patients actively seek health information online, aligning perfectly with decentralized trials that use technology to reduce patient burden and accelerate the development of new treatments.
At the heart of this revolution are AI and federated data platforms. They enable the secure analysis of vast real-world data to find the right patients with incredible accuracy and optimize campaigns in real-time, all while maintaining the strictest privacy and governance. This smart technology is changing clinical research.
Lifebit is proud to lead this shift. Our federated AI platform provides the secure, real-time access to global biomedical data that makes these successes possible. With tools for data harmonization, advanced AI/ML analytics, and secure governance, we empower researchers with real-time insights and secure collaboration.
The era of guesswork in recruitment is over. The future is digital and data-driven, paving the way for faster, more inclusive, and more impactful clinical research.
Request a demo of our real-time evidence and analytics solutions.


