Drug Discovery Demystified: How New Medicines Make It to Market

Drug discovery and development: 5 Breakthrough Stages
Why Understanding Drug Findy and Development Matters
Drug findy and development is the complex scientific process that transforms promising compounds into life-saving medicines. This multi-stage journey involves identifying therapeutic targets, screening thousands of potential compounds, conducting rigorous clinical trials, and navigating regulatory approval—all while ensuring patient safety and treatment efficacy.
The stakes are incredibly high. It takes approximately 12-15 years and costs around $2.8 billion to bring a single new medicine to market. Despite this massive investment, the vast majority of compounds tested never reach patients. Yet, successful medicines can transform lives and address critical unmet medical needs for millions globally.
The pharmaceutical industry continues to grow rapidly, with global drug sales expected to reach $1.9 trillion by 2027. This growth is driven by aging populations, emerging diseases, and breakthrough technologies like artificial intelligence that are revolutionizing how we find and develop new treatments.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years at the intersection of computational biology, AI, and drug findy and development, my work with our federated genomics platform has provided deep insights into the challenges and opportunities shaping the future of pharmaceutical innovation.
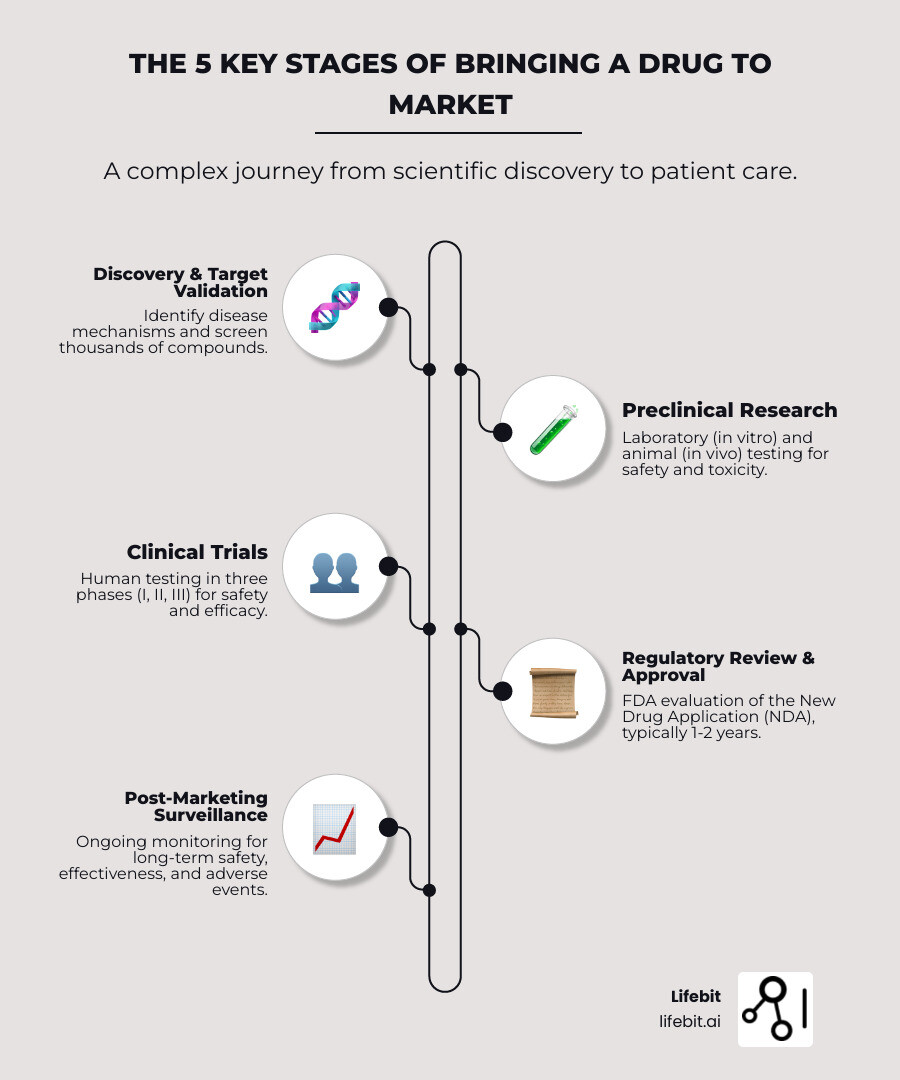
The 5 Key Stages of Bringing a Drug to Market
Getting a new medicine from a lab to a pharmacy is a high-stakes journey. Every step, from the initial idea to rigorous testing and regulatory review, is designed to ensure new medicines are safe and effective. It’s a path filled with scientific challenges, ethical considerations, and significant financial investment. Let’s explore The journey of a medicine.
Stage 1: Findy and Target Validation
The journey begins with drug findy. The first step is target identification, where scientists study a disease’s biological mechanisms to find specific targets—like genes, proteins, or enzymes—that play a key role in its progression. This process leverages fields like genomics (studying the genome), proteomics (studying proteins), and bioinformatics to pinpoint molecules that can be modulated to alter the disease course. A viable target must be effective, safe, and “druggable,” meaning a therapeutic molecule can be designed to bind to it and produce a desired effect.
Next is target validation, which uses techniques like gene editing (e.g., CRISPR) or RNA interference to confirm that modulating the target will have a positive, therapeutic effect on the disease. Once validated, the hunt for lead identification begins. This involves screening vast chemical libraries, sometimes containing millions of molecules, to find “hits” that interact with the target. Key methods include:
- High-Throughput Screening (HTS): Automated testing of large numbers of compounds to quickly identify active hits.
- Fragment-Based Drug Findy (FBDD): Screening smaller, simpler chemical fragments that bind weakly to the target and then growing or combining them into a more potent lead compound.
- Structure-Based Drug Design (SBDD): Using the known 3D structure of a target protein to design molecules that fit perfectly into its active site.
For every 10,000 compounds tested, only 10 to 20 may advance. These hits undergo lead optimization, where medicinal chemists systematically modify their structure to improve potency, selectivity, and drug-like properties. This iterative process, guided by Structure-Activity Relationship (SAR) studies, aims to maximize efficacy while minimizing potential toxicity and addressing any intellectual property issues early.
Therapeutic agents generally fall into three main types:
- Small Molecules: Chemically synthesized compounds, often taken orally as pills (e.g., ibuprofen).
- Biologics: Large, complex molecules from living organisms, such as proteins or vaccines, usually administered by injection (e.g., insulin).
- Gene Therapy: A cutting-edge approach that modifies a person’s genes to treat or cure a disease.
Stage 2: Preclinical Research
Promising lead compounds move to preclinical research to evaluate safety and efficacy before human testing. This stage involves both lab experiments (in vitro) and animal studies (in vivo).
- In vitro studies: Experiments conducted in a controlled lab environment (e.g., test tube, cell culture) to assess a compound’s biological activity and initial toxicity.
- In vivo animal testing: Studies performed on living organisms to see how a drug affects a whole biological system and to gather essential safety data.
Preclinical research focuses on a drug’s pharmacokinetics—what the body does to the drug (ADME: Absorption, Distribution, Metabolism, and Excretion)—and its toxicology to identify potential harmful effects. Toxicology studies are extensive, assessing acute toxicity (single high dose), chronic toxicity (long-term exposure), carcinogenicity (cancer-causing potential), and reproductive toxicity. All studies must follow strict Good Laboratory Practices (GLP) to ensure data quality for regulatory bodies like the U.S. Food and Drug Administration (FDA). While animal models are crucial, they don’t always perfectly predict human responses. The FDA Modernization Act 2.0 (2022) now allows new drugs to potentially bypass animal testing if sufficient data from alternative methods is available. These alternatives include advanced in vitro models like organoids (mini-organs) and organ-on-a-chip technologies, paving the way for more efficient and ethical development.
Stage 3: Clinical Trials
After successful preclinical research, an Investigational New Drug (IND) application is submitted to regulators. Once approved, clinical trials—rigorous human testing phases—can begin. All trials require approval from an Institutional Review Board (IRB) to ensure they are ethical and that participants provide informed consent.
Clinical trials are typically divided into four main phases:
- Phase I: The drug is given to a small group of 20-80 healthy volunteers to assess its safety profile, determine a safe dosage range, and study its pharmacokinetics and pharmacodynamics (what the drug does to the body). About 70% of drugs pass this phase.
- Phase II: The drug is tested in a larger group of 100-500 patients with the target condition. This phase aims to evaluate efficacy (does the drug work?) and establish “proof-of-concept.” It also involves dose-ranging studies to find the optimal dose and further assesses safety by identifying common side effects. Only about 33% of drugs advance from Phase II, making it a critical hurdle.
- Phase III: This large-scale phase involves 1,000-5,000 patients across multiple global centers. These are often randomized controlled trials (RCTs) where the new drug is compared against a placebo or the existing standard of care. Phase III trials are designed to definitively confirm efficacy, monitor for adverse reactions in a larger population, and provide the primary evidence for regulatory approval. Only 25-30% of drugs in Phase III proceed to the next stage. Overall, just 12% of drugs that enter clinical trials reach Phase III.
Stage 4: FDA Review and Approval
After Phase III, all data is compiled into a New Drug Application (NDA) for small molecules or a Biologics License Application (BLA) for biologics. This comprehensive submission includes all preclinical and clinical data, information on the drug’s chemistry and manufacturing processes, and the proposed labeling. The regulatory body (e.g., the FDA in the U.S., the EMA in Europe) then conducts an extensive regulatory review, which typically takes 1-2 years. Experts assess if the drug’s benefits outweigh its risks (risk-benefit analysis) and inspect manufacturing facilities to ensure they meet quality standards (Good Manufacturing Practices).
To expedite access to important new medicines, the FDA offers several programs:
- Fast Track: For drugs treating serious conditions and filling an unmet medical need.
- Breakthrough Therapy: For drugs showing substantial improvement over available therapy on a clinically significant endpoint.
- Priority Review: Reduces the FDA’s review goal from 10 months to 6 months.
- Accelerated Approval: Allows for earlier approval of drugs that treat serious conditions based on a surrogate endpoint.
If approved, the drug can be marketed with detailed labeling and prescribing information.
Stage 5: Post-Marketing Surveillance (Phase IV)
After approval, post-marketing surveillance begins. This is often called a Phase IV trial and involves ongoing monitoring of the drug’s safety and effectiveness in a large, real-world patient population. This phase is crucial for detecting rare or long-term side effects not seen in the controlled environment of clinical trials. The practice of monitoring for adverse drug events is known as pharmacovigilance. Data is collected through systems like the FDA’s Adverse Event Reporting System (FAERS) and from real-world evidence (RWE) gathered from electronic health records and insurance claims. Sometimes, new uses for the drug are finded during this phase—for example, minoxidil was originally a blood pressure drug before its hair-growth side effect led to its repurposing as Rogaine. Citizen science initiatives are also emerging as valuable tools for gathering broad, real-world insights to improve patient outcomes.
Innovations Accelerating the Drug Findy and Development Pipeline
The traditional drug findy and development pipeline is lengthy and expensive, creating a critical need for innovations to accelerate the process, reduce costs, and improve success rates. New technologies and approaches are making drug development faster, more precise, and more efficient.
Drug Repurposing: New Tricks for Old Drugs
Drug repurposing (or repositioning) is a strategy that involves finding new therapeutic uses for existing, approved drugs. The advantages are significant: since the safety, dosing, and pharmacokinetic data are already known, development timelines and costs can be drastically reduced. Repurposed drugs can often bypass early-stage development and move directly into Phase II clinical trials.
Approaches to drug repurposing can be:
- Computational: Using AI and machine learning to mine vast databases of genetic, molecular, and clinical data to predict new drug-disease connections.
- Experimental: Systematically screening libraries of existing drugs against new disease targets.
Classic examples include sildenafil, originally developed for angina and repurposed as Viagra for erectile dysfunction, and thalidomide, repurposed for leprosy and multiple myeloma. A more recent example is fenfluramine, now used for rare forms of epilepsy. A notable industry case is UCB’s acquisition of Zogenix Inc, driven by its repurposed drug Fintepla. However, challenges remain, including securing intellectual property (often through new method-of-use patents), determining the correct dosage for the new indication, and ensuring commercial viability.
The Role of Artificial Intelligence (AI) in Drug Findy and Development

Artificial Intelligence (AI) is rapidly changing drug findy and development. AI uses powerful algorithms to analyze vast volumes of data, accelerating multiple stages of the pipeline:
- Target Identification: AI can analyze genomic, proteomic, and clinical data to identify novel disease targets that human researchers might miss.
- Drug Design and Optimization: Generative AI models can design entirely new molecules (de novo drug design) with specific desired properties, dramatically speeding up the lead optimization process.
- Preclinical Research: AI can predict a compound’s toxicity and pharmacokinetic properties, helping to prioritize candidates and reduce reliance on animal testing.
- Clinical Trial Optimization: AI can help design more efficient trials, identify ideal patient populations for recruitment, and analyze trial data in real time to predict outcomes.
A prime example is the compound DSP-1181, an AI-developed drug whose initial development was 4 times faster than conventional methods. Although it ultimately failed in Phase I trials, it demonstrated AI’s potential to dramatically compress early timelines. AI-finded drugs do not guarantee clinical success; critical thinking and data understanding remain essential. Despite its promise, AI in this field faces limitations. Models are dependent on high-quality, well-structured data, which can be scarce. The biological complexity of living systems is difficult to fully model, and the “black box” nature of some AI predictions can make it hard for scientists to gain insights. The concept of “digital twins”—virtual models of patients to simulate drug effects—is also being explored to reduce the need for physical testing.
Platform Technologies: Building a Foundation for Rapid Innovation
Another key innovation is the rise of platform technologies. These are standardized systems or frameworks that can be used to develop multiple drugs for different diseases. By creating a foundational “chassis,” companies can rapidly swap in new genetic information or targets to create new therapeutic candidates, saving immense time and resources.
- mRNA Platforms: The COVID-19 pandemic showcased the power of mRNA technology. Companies like Moderna and Pfizer/BioNTech were able to develop highly effective vaccines in under a year because the underlying mRNA delivery platform was already established. This platform can now be adapted to target other infectious diseases and even cancers.
- AAV Platforms: In gene therapy, Adeno-Associated Virus (AAV) is often used as a vector to deliver genetic material into cells. By developing a reliable AAV platform, companies can create a pipeline of gene therapies for various genetic disorders by simply changing the genetic payload.
Careers and Education in the Pharmaceutical Industry
The dynamic field of drug findy and development is a rapidly growing sector with diverse and rewarding career opportunities. The industry has a high demand for skilled professionals across many disciplines.
Career Opportunities and Salary Expectations
A career in drug findy and development can lead to roles in pharmaceutical companies, biotech firms, contract research organizations (CROs), and government agencies. These positions require a strong scientific foundation, analytical skills, and knowledge of regulatory affairs. Here are some common career paths and their average annual salaries:
- Medical Scientist: Conducts research to improve human health. The mean wage was $112,380 in May 2023, rising to $121,230 for those in scientific research positions.
- Drug Development Scientist: Focuses on preclinical and clinical studies. Average salary: $111,245. (Glassdoor.com)
- Pharmacologist: Studies how medicines interact with the body. Average salary: $110,136. (Indeed.com)
- Drug Findy Scientist: Identifies and optimizes new compounds. Average salary: $104,756. (Salary.com)
- Clinical Research Associate (CRA): Manages and monitors clinical trials. Average salary: $93,292. (Indeed.com)
These roles demand a blend of scientific expertise, problem-solving abilities, and strong communication skills.
Educational Pathways for a Career in Drug Findy and Development
Various educational pathways can prepare you for a career in drug findy and development.
- Professional Certificates: These focused programs (6-12 months) provide a foundation in the core principles of the field, from early findy to regulatory approval. They are ideal for career advancement or for those new to the industry.
- Master’s Degrees: An MS in Drug Findy and Development offers in-depth knowledge and practical skills, often with flexible online options for working professionals. These programs (1-3 years) focus on industry-relevant skills through capstone projects or internships.
- PhD Programs: For those aiming to lead cutting-edge research, a Ph.D. in pharmacology, medicinal chemistry, or a related field involves extensive research to advance scientific understanding.
These pathways equip you with specialized knowledge, analytical skills, regulatory understanding, and valuable networking opportunities.
The Future of Drug Development
Drug findy and development is advancing toward a future of highly precise and personalized treatments, driven by scientific breakthroughs and a deeper understanding of human biology.
Personalized Medicine, also known as precision medicine, aims to tailor medical treatment to each patient’s unique characteristics. This approach moves beyond a one-size-fits-all model by using genetic, environmental, and lifestyle data to guide prevention, diagnosis, and treatment. A key component is the use of companion diagnostics, which are tests that identify patients most likely to benefit from a specific therapy. This can help predict drug responses, reduce adverse side effects, and optimize treatment outcomes. A prime example is Precision Oncology, which develops cancer treatments targeting specific genetic mutations in a patient’s tumor. For instance, drugs like Herceptin are highly effective for breast cancers that overexpress the HER2 protein, while EGFR inhibitors are used for lung cancers with specific EGFR mutations.
We are also seeing incredible progress in Advanced Therapy Medicinal Products (ATMPs), an innovative group of treatments that includes:
- Gene Therapy: Directly modifying a patient’s genes to treat or cure diseases. This can be done ex vivo (cells are removed, modified, and returned to the body) or in vivo (a vector, like an Adeno-Associated Virus or AAV, delivers the gene directly into the body). A landmark example is Zolgensma, a one-time treatment for spinal muscular atrophy (SMA).
- Cell Therapy: Using living cells to fight disease. The most prominent example is CAR T-cell therapy, where a patient’s own T-cells are engineered to recognize and attack cancer cells. Other approaches involve using stem cells to repair or replace damaged tissue.
- RNA Therapeutics: Using RNA molecules to modulate gene expression. This includes mRNA vaccines (which instruct cells to produce a target protein) and RNA interference (RNAi) drugs, which can “silence” specific genes responsible for causing disease, offering new ways to tackle previously untreatable conditions.
Furthermore, open science collaborations between academia, industry, and non-profits are breaking down barriers and accelerating findies. By sharing data, tools, and knowledge pre-competitively, these partnerships tackle fundamental research challenges that no single organization could solve alone. A leading example is the Structural Genomics Consortium (SGC), which makes 3D protein structures freely available to the global research community, helping to open up new drug targets. This collaborative spirit helps bring new, life-changing therapies to patients faster.
As we look ahead, it’s important to understand the differences between major drug types, particularly regarding their manufacturing and cost.
| Feature | Small Molecules | Biologics |
|---|---|---|
| Composition | Chemically synthesized organic compounds | Large, complex molecules from living organisms |
| Size | Small (low molecular weight) | Large (high molecular weight) |
| Administration | Often oral (pills, capsules) | Typically injectable (infusions, injections) |
| Manufacturing | Chemical synthesis | Biological processes (cell cultures) |
| Cost (Daily Dose) | Generally lower | Significantly higher (22 times more on average) |
| Immunogenicity | Low | Higher potential to trigger immune response |
| Examples | Aspirin, Lipitor | Insulin, Monoclonal Antibodies, Vaccines |
You can learn more about the differences between biologics and small molecules here.
While biologics are revolutionary, their higher cost—an average daily dose can be 22 times more than a small molecule—raises important questions about drug pricing and access that will continue to shape the future of drug findy and development.
Frequently Asked Questions about Drug Development
Here are answers to some of the most common questions about the complex process of drug findy and development.
How much does it really cost to develop a new drug?
The estimated cost to develop a new medicine is around US $2.8 billion. This figure includes not only the direct R&D expenses for the single successful drug but also the immense costs of the many promising compounds that fail during preclinical or clinical testing. The expenses of large-scale clinical trials and manufacturing also contribute significantly to the high cost.
Why does it take so long to get a new drug approved?
The drug findy and development process typically takes 12–15 years. This lengthy timeline is a result of the rigorous, sequential stages required to ensure patient safety and efficacy. Each phase, from initial findy to final clinical trials and regulatory review (which alone can take one to two years), involves extensive testing and data collection. This methodical approach is essential for protecting public health.
What percentage of drugs that start preclinical testing make it to market?
The pharmaceutical industry has a very high attrition rate. For every 10,000 compounds initially screened, only a handful advance to formal development. The likelihood of a drug that enters clinical trials making it all the way to Phase III is just 12%, and even fewer receive final approval. This high failure rate, often called the “valley of death,” highlights the challenges of drug development, but each failure provides valuable insights that drive future innovation.
Conclusion
The journey of drug findy and development is a complex, lengthy, and expensive endeavor, filled with high risks and immense challenges. Yet, it is also a field of tremendous reward, constantly pushing scientific boundaries to address critical medical needs and improve global health. Every step, from the initial lab findy to a life-changing medicine reaching patients, is a testament to human ingenuity and perseverance.
Looking ahead, the landscape of drug findy and development will continue to evolve rapidly, driven by scientific breakthroughs and technology. Artificial Intelligence, in particular, holds transformative potential to accelerate every stage of the process, improve predictive accuracy, and bring innovative treatments to patients faster.
At Lifebit, we are at the forefront of this revolution. Our next-generation federated AI platform enables secure, real-time access to global biomedical data. With built-in tools for data harmonization, advanced AI/ML analytics, and secure governance, we empower organizations to conduct large-scale, compliant research and monitor drug safety with unprecedented efficiency.
Our platform, featuring components like the Trusted Research Environment (TRE) and our Real-time Evidence & Analytics Layer (R.E.A.L.), delivers immediate insights and AI-driven safety surveillance. We believe that by leveraging these powerful tools, we can collectively accelerate findies and speed up the delivery of essential medicines to those who need them most.
Learn more about Lifebit’s Real-time Evidence & Analytics Layer (R.E.A.L.)


