EDC Systems Unplugged: How Clinical Research is Going Digital

EDC in clinical research: The Ultimate 2025 Guide
The Digital Revolution in Clinical Research
EDC in clinical research has transformed data collection and management in clinical trials. At its core, Electronic Data Capture (EDC) software replaces paper case report forms with digital versions, enabling real-time data entry, automated validation, and secure storage.
Key aspects of EDC in clinical research:
- What it is: Web-based software that digitizes clinical trial data collection
- Who uses it: Research sites, sponsors, CROs, and data managers
- Main benefits: Improved data quality, faster timelines, improved compliance
- Core features: Electronic case report forms (eCRFs), automated edit checks, audit trails
- Data types: Patient demographics, vital signs, lab results, adverse events, medications
The numbers tell the story. A 2021 analysis found Phase III trials generate 3.6 million data points on average—three times more than a decade ago. With 80 % of trial sites using digital technologies as of 2022, the industry has moved far beyond paper.
This data explosion makes EDC systems essential. They are no longer just digital forms but comprehensive platforms that integrate with labs, imaging systems, wearables, and electronic health records.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in computational biology and AI, I’ve seen EDC in clinical research evolve from simple data capture tools into the intelligent, federated systems that power the future of drug findy.
Basic edc in clinical research vocab:
Explaining Electronic Data Capture (EDC): The Fundamentals
EDC in clinical research acts as the digital nervous system for a clinical trial. It gathers data from multiple sources and funnels it into a centralized, secure hub for analysis and action.
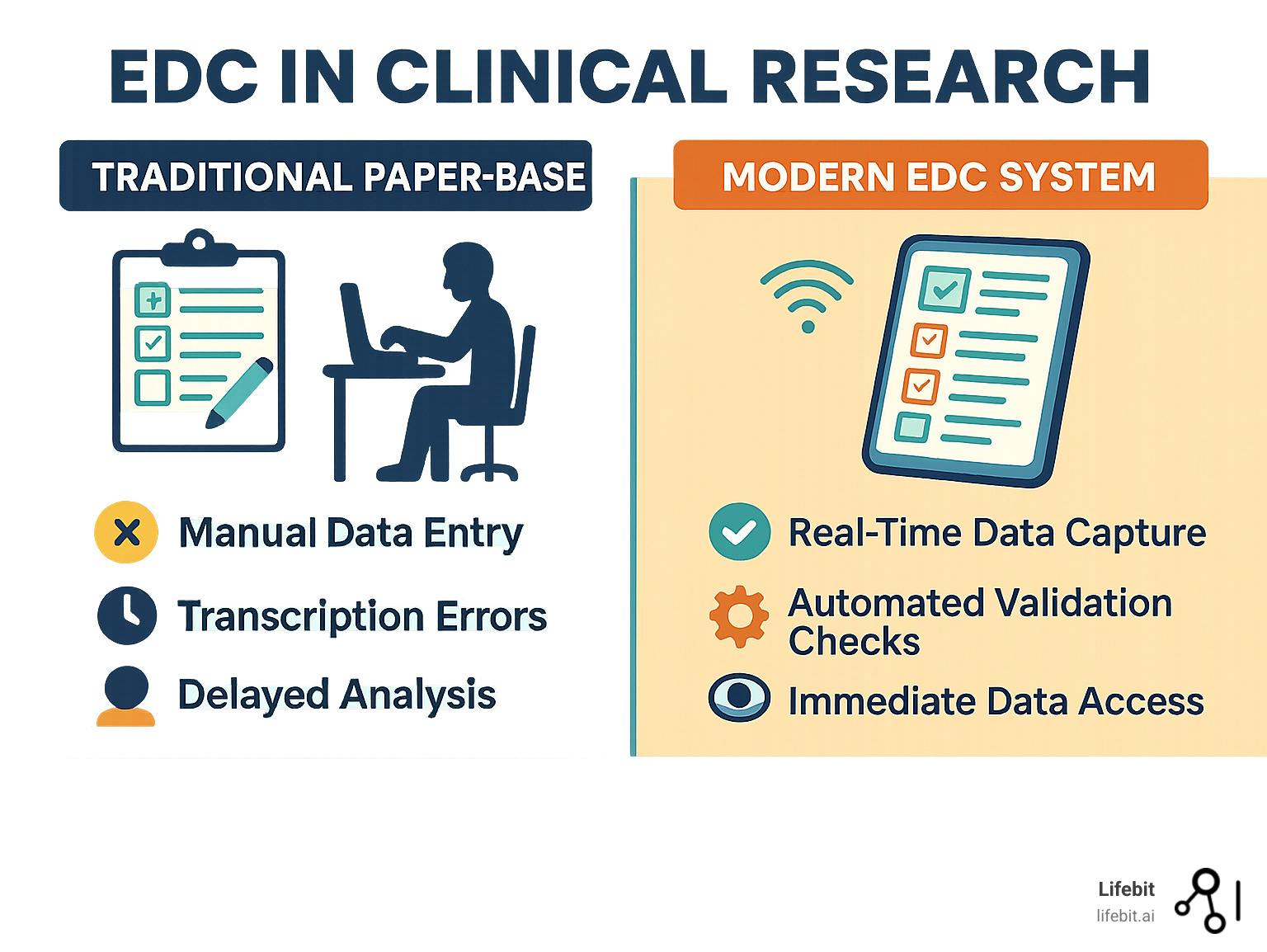
An EDC system is a software application that streamlines clinical trials by digitizing Case Report Forms (CRFs). Instead of investigators transcribing information from paper source documents onto paper CRFs—a process fraught with potential for illegibility and transcription errors—they use interactive electronic CRFs (eCRFs). This data is captured and stored in a centralized, web-accessible database, making it instantly available to the entire study team, regardless of their location.
The change is revolutionary, like comparing mail by horseback to instant messaging. It transforms data collection from a slow, linear, and error-prone process into a dynamic, real-time, and high-quality operation.
| Metric | Traditional Paper-Based Data Collection | Modern EDC Systems |
|---|---|---|
| Data Quality | Prone to transcription errors, illegibility, and missing data. | Improved accuracy via built-in validation checks, auto-calculations, and real-time error detection. |
| Speed to Database Lock | Slow due to manual data entry, extensive cleaning, and query resolution. | Significantly faster with real-time entry, automated queries, and streamlined cleaning. |
| Cost | High administrative costs for printing, shipping, storage, and manual data entry/cleaning. | Higher initial setup costs, but significant long-term savings in labor, materials, and reduced trial duration. |
| Accessibility | Limited to physical documents; delayed access for remote teams. | Real-time, remote access for authorized users globally, enabling faster decision-making. |
| Security | Vulnerable to physical loss, damage, and unauthorized access. | Robust digital security measures, audit trails, and access controls for data integrity and confidentiality. |
What is an EDC system in clinical research?
An EDC in clinical research system is a trial’s command center, managing the data lifecycle from capture to submission. Its core is the Electronic Case Report Form (eCRF), an intelligent digital questionnaire. Unlike static paper forms, eCRFs can validate data in real-time, catching errors at the moment of entry. The EDC system is not a single entity but a suite of integrated modules. The eCRF Designer allows study builders to create forms using drag-and-drop interfaces, often leveraging pre-built libraries of standard forms (e.g., demographics, vitals) to accelerate setup. The Data Validation Engine is the system’s logical core, running automated edit checks in real-time. These can range from simple range checks (e.g., heart rate must be between 30 and 250) to complex cross-form logic (e.g., if a patient is marked as male, the pregnancy test form should be disabled). The Query Management Module automates the process of identifying, assigning, and resolving data discrepancies. When an edit check fails, a query is automatically generated and assigned to the appropriate site user for resolution. The entire lifecycle of the query—from creation to answer to closure—is tracked. Finally, the Reporting and Analytics Module provides real-time visibility into trial metrics, such as enrollment rates per site, data entry timeliness, and the number of open queries, allowing for proactive trial management. With secure storage and a complete audit trail, authorized team members can analyze information as it arrives, confident in its integrity.
This real-time capability is a game-changer, allowing teams to spot trends, safety signals, or enrollment issues immediately instead of waiting weeks for paper forms to be collected and entered. For deeper insights, see this Comparison of Electronic Data Capture with Paper Data Collection – Is There Really an Advantage? and our guide on Clinical Research Data Software.
Who Uses EDC Systems in the Clinical Trial Ecosystem?
The beauty of EDC in clinical research is how it connects everyone involved in a clinical trial into a collaborative, data-driven ecosystem.
- Clinical research sites: Front-line users like investigators and study coordinators use the EDC as their primary tool for data submission. This includes entering patient data during visits, responding to automated queries, and providing electronic signatures to attest to the data’s accuracy. A well-designed EDC minimizes their administrative burden, allowing them to focus more on patient care.
- Sponsors: Pharma, biotech, and medical device firms rely on EDC systems to monitor trial progress and quality in real-time. They use dashboards to monitor Key Performance Indicators (KPIs) and Key Risk Indicators (KRIs), enabling data-driven decisions about site performance, patient safety, and overall trial strategy.
- Contract Research Organizations (CROs): CROs are often power users of EDC systems. Data Managers use the system’s advanced tools to perform data review, manage complex queries, and prepare the dataset for analysis. Clinical Research Associates (CRAs) use the EDC for remote monitoring and Source Data Verification (SDV), reducing the need for costly on-site visits.
- Patients: Patients are increasingly direct users via integrated electronic Patient Reported Outcomes (ePRO) tools. They use their own smartphones or provisioned devices to log symptoms, report on quality of life, or confirm medication adherence. This direct-from-patient data stream is invaluable for capturing subjective endpoints.
- Statisticians and Medical Monitors: Statisticians access cleaned data exports for interim and final analyses, while medical monitors review patient profiles and safety data in real-time to ensure patient well-being and data integrity.
Clearing Up the Acronyms: EDC vs. eCRF, CDMS, and CTMS
Clinical research has many acronyms. Here’s how EDC in clinical research relates to other key systems.
- EDC vs. eCRF: EDC (Electronic Data Capture) is the overall platform or system. The eCRF (Electronic Case Report Form) is a core component within it—the digital form used for capturing participant data for a specific visit or event. An eCRF cannot exist without an EDC system to host it, validate its data, and manage its lifecycle.
- EDC vs. CDMS: The line between EDC and CDMS (Clinical Data Management System) has blurred significantly. Historically, a CDMS was a separate system used to manage, clean, and analyze data after it was collected. Today’s modern EDC systems have incorporated these data handling and management features, so the terms are often used interchangeably to refer to the same platform.
- EDC vs. CTMS: A CTMS (Clinical Trial Management System) serves a completely different purpose. It focuses on the operational and logistical management of a trial, not the patient-specific clinical data. A CTMS tracks things like site contact information, IRB submission dates, patient enrollment numbers, study milestones, and financial budgets. It is the project management software for the trial, while the EDC is the data collection tool.
- EDC vs. ePRO/eCOA: Electronic Patient-Reported Outcomes (ePRO) and Electronic Clinical Outcome Assessments (eCOA) are specialized tools for capturing data directly from patients, caregivers, or clinicians, often on tablets or smartphones. While sometimes standalone, they are increasingly integrated directly into the EDC platform. The EDC acts as the central repository for this data, combining it with site-entered data to create a complete patient record.
The real power comes from system integration. When an EDC automatically feeds enrollment data into a CTMS, it creates a seamless ecosystem that reduces redundant data entry, eliminates manual work, and provides sponsors with a holistic view of both clinical and operational performance.
The Core Benefits of Using EDC in Clinical Research
Switching to EDC in clinical research is more than just swapping paper for pixels. It’s a fundamental operational shift that yields significant improvements across the entire clinical trial lifecycle, from data quality and patient safety to the speed of bringing new treatments to patients.
The numbers are compelling. Studies have shown that EDC can reduce data error rates by up to 70% and shorten trial timelines by an average of 30%. This translates directly into faster market access for new therapies, reduced development costs, and more reliable data for making critical decisions.
Let’s dig deeper into how EDC in clinical research delivers these transformative benefits.
Boosting Data Quality and Accuracy
On paper, data quality is constantly at risk from illegible handwriting, missing fields, and transcription errors. EDC in clinical research systems are designed to systematically eliminate these issues at the source.
- Automated edit checks: These are programmed rules that act as a vigilant assistant, instantly flagging impossible or inconsistent values. For example, an edit check can prevent a user from entering a date of birth that indicates the patient is 250 years old (a range check) or flag an entry for a pregnancy test result for a patient registered as male (a logical check).
- Data validation rules: These ensure consistency across the entire dataset, such as verifying that a medication’s end date does not precede its start date.
- Reduced transcription errors: Direct data entry into the EDC system eliminates the error-prone step of transcribing information from paper source documents to a paper CRF, a major source of errors in traditional trials.
- Query management: The built-in system streamlines issue resolution. When an edit check fails, a query is automatically generated, the site coordinator is notified, they provide a correction or clarification, and a data manager reviews and closes the query. This structured process eliminates the messy, untraceable back-and-forth of sticky notes and emails common with paper-based trials, ensuring the final dataset is robust and defensible.
The result is cleaner data for submission that regulatory agencies can trust, reducing the number of questions during review. This focus on data quality is explored further in our article on Data Harmonization: Overcoming Challenges.
Accelerating Trial Timelines and Efficiency
In EDC in clinical research, time operates differently. Processes that took weeks or months on paper now happen in near real-time.
- Real-time data access: Information entered at a site in one country is instantly available to the global trial team, eliminating shipping delays and lost paperwork.
- Remote monitoring: A key driver of this efficiency is the shift towards Risk-Based Monitoring (RBM), a strategy endorsed by regulatory bodies like the FDA and EMA. Instead of performing 100% Source Data Verification (SDV) on every data point for every patient—a time-consuming and expensive process—RBM uses the real-time data in the EDC to focus monitoring efforts on the most critical data and processes. Central monitors can analyze data trends across sites to identify anomalies that may indicate poor training, protocol deviation, or even fraud. This allows on-site CRAs to conduct more targeted, efficient visits, focusing their attention where it’s needed most.
- Faster data cleaning and review: With automated checks handling the simple errors, data managers can focus their expertise on complex clinical questions and data trends.
- Quicker database lock: All of these efficiencies culminate in a significantly quicker database lock—the critical milestone where the data is declared final and ready for statistical analysis. This can shorten the overall trial timeline by months.
- Reduced site burden: Remote capabilities and streamlined data entry reduce the administrative workload and travel costs for research sites, improving site satisfaction and engagement.
These gains are part of broader trends we explore in Emerging Trends & Technologies in Clinical Trial Design.
Enhancing Regulatory Compliance and Data Security
In the highly regulated environment of clinical research, trust and traceability are paramount. EDC in clinical research systems are specifically engineered to establish and maintain that trust with regulatory bodies.
- Comprehensive audit trails: Every action in the EDC—every data point entered, corrected, or deleted—is recorded in an unalterable, computer-generated audit trail. This log shows the what, who, when, and why of every change, ensuring complete transparency and accountability.
- User access controls: Granular, role-based permissions ensure that users can only see and modify data relevant to their specific role, enhancing security and preventing unauthorized actions.
- Data encryption: All sensitive patient information is protected by strong encryption, both during transmission over the internet (in transit) and while stored in the database (at rest).
- HIPAA and GDPR compliance: For 21 CFR Part 11, this means the EDC system must have features like secure, time-stamped audit trails; controls to ensure electronic signatures are the legal equivalent of handwritten signatures; and strict user authentication protocols. Beyond the US, global regulations like the General Data Protection Regulation (GDPR) in Europe impose strict rules on handling personal data. EDC systems support GDPR compliance through features like role-based access controls that enforce data minimization and data encryption.
- 21 CFR Part 11 compliance: This FDA regulation sets the standard for ensuring that electronic records are as trustworthy and reliable as paper records. Compliant EDC systems provide superior security, data integrity, and traceability.
Key Features and Implementation Considerations
Modern EDC in clinical research systems are sophisticated, multi-faceted platforms, not just digital forms. When selecting and implementing a system, sponsors must look beyond a simple feature checklist and consider user experience, scalability, interoperability, and vendor support. These factors ultimately determine whether a system becomes a powerful enabler for the research team or a frustrating hindrance.
Essential Features of a Modern EDC Platform
The best EDC in clinical research platforms share certain essential features that drive efficiency and quality:
- Intuitive eCRF Designer: A user-friendly, drag-and-drop interface that allows clinical data managers and study builders to create and deploy forms quickly, without needing extensive programming skills. The ability to reuse forms and libraries across studies is a key efficiency driver.
- Automated Query Management: The system should not only flag data inconsistencies but also automate the workflow of assigning, tracking, and resolving queries in a closed-loop process.
- Advanced Reporting and Analytics: Real-time, customizable dashboards are essential. They provide immediate insights into study progress, enrollment trends, data quality metrics, and site performance, enabling proactive management.
- Role-Based Access Control: The ability to configure precise user permissions is fundamental to security and usability, ensuring that investigators, data managers, and monitors only access the data and functions appropriate for their role.
- Third-Party Data Integration (API): Robust Application Programming Interfaces (APIs) are crucial for modern trials. They allow the EDC to connect seamlessly with external systems like central labs, imaging vendors, EHRs, and other clinical systems, automating data flow and eliminating manual data reconciliation.
- Integration with RTSM/IWRS: Seamless integration with Randomization and Trial Supply Management (RTSM), also known as Interactive Web Response Systems (IWRS), is critical. This allows the EDC to automatically trigger patient randomization or dispense medication kits once eligibility criteria entered into the eCRFs are met, eliminating manual steps and potential errors.
- Medical Coding Tools: Integrated tools for coding adverse events and medications against standard dictionaries like MedDRA and WHODrug ensure consistency and prepare the data for analysis and submission.
Planning for Success: Implementation Challenges
Successful implementation of EDC in clinical research requires careful planning to steer potential challenges:
- Initial setup costs and study build time: While EDC delivers long-term ROI, initial costs for licensing and setup can be significant, ranging from $15,000 for small, simple trials to over $5 million for large, complex Phase III programs. The study build process—which includes protocol review, eCRF specification, form building, edit check programming, and user acceptance testing (UAT)—requires a dedicated upfront investment of time but pays dividends later by minimizing errors and data cleaning efforts.
- Staff training and adoption: To overcome resistance to new technology, it is vital to choose an intuitive system and provide comprehensive, role-based training. A robust change management plan that communicates the benefits and provides strong support is key to ensuring smooth adoption by site staff and the study team.
- Data migration planning: When moving from a legacy system or paper, a detailed data migration plan is essential. This involves careful data mapping, validation, and quality control to ensure no information is lost or corrupted in the transfer.
- Choosing the right vendor: This is a strategic decision. Look beyond the feature list to consider the vendor’s regulatory compliance track record, experience in your specific therapeutic area, data standardization support (e.g., CDISC), and the quality and responsiveness of their ongoing customer and technical support.
The Importance of Data Standards in EDC
In EDC in clinical research, data standards are the universal translators that ensure data can be understood, shared, and analyzed consistently across different systems and studies. The standards developed by the Clinical Data Interchange Standards Consortium (CDISC) are essential, as regulatory bodies like the FDA and Japan’s PMDA now require or strongly recommend that submission data be in these formats.
- CDASH (Clinical Data Acquisition Standards Harmonization): Standardizes how data is collected on the eCRFs, ensuring that common data points are captured the same way across all studies.
- SDTM (Study Data Tabulation Model): Provides a standard structure for organizing and formatting clinical trial data for regulatory submission.
- ADaM (Analysis Data Model): Standardizes the creation of analysis datasets that are ‘ready for analysis’ and support traceability from data collection to results.
Adherence to these standards is not merely a technical exercise; it has profound practical implications. When data is collected and structured according to CDISC standards from the outset, it dramatically streamlines the process of preparing a regulatory submission. FDA reviewers can use standardized tools to analyze the data more efficiently, potentially shortening review cycles. Furthermore, standardized data is more easily aggregated for meta-analyses, making the data FAIR—Findable, Accessible, Interoperable, and Reusable.
The Evolution and Future of Clinical Data Capture
The journey of EDC in clinical research is a story of continuous technological evolution. From cumbersome, isolated systems to intelligent, interconnected cloud platforms, each leap has built upon the last, creating the powerful tools that are now indispensable for modern clinical trials.

From Remote Data Entry to the Cloud: A Brief History
The first attempts at electronic data capture in the late 1980s and early 1990s were known as Remote Data Entry (RDE). These systems used bulky desktop computers with locally installed, proprietary software. Data was entered offline at the clinical site and then transmitted periodically to a central server via a modem. While innovative for its time, this model was a logistical nightmare. It required specialized hardware and IT support at each site, leading to high costs, inconsistency, and significant deployment challenges.
The rise of the commercial internet in the late 1990s ushered in the era of web-based EDC, a game-changer that eliminated the need for site-specific software. Any computer with a standard web browser could access the central system, dramatically slashing costs, simplifying deployment, and improving accessibility. Today, the dominant model is Software-as-a-Service (SaaS), where cloud-based EDC systems are developed, hosted, and managed by third-party vendors. This model offers superior scalability, security, and accessibility, removing the IT infrastructure burden from sponsors and allowing them to focus on the science of the trial.
The Rise of eSource: Capturing Data at the Point of Origin
Even with web-based EDC, a significant amount of data was still being transcribed—copied from a paper source document (like a patient chart) into the eCRF. eSource, a concept heavily promoted by the FDA’s eSource Guidance in 2013, aims to solve this inefficiency by capturing data electronically at its point of origin, eliminating transcription entirely.
Key eSource methods include direct data capture from EHRs, where clinical data flows automatically into the EDC, and the use of wearables and sensors to continuously collect objective data like activity levels, sleep patterns, and heart rates. This passive data collection provides a much richer, more realistic picture of a patient’s health than the snapshots captured during infrequent clinic visits. However, the promise of seamless eSource integration, particularly from EHRs, faces significant problems. The primary challenge is a lack of interoperability; EHR systems from different vendors often store the same information in different formats. The emergence of standards like HL7’s Fast Healthcare Interoperability Resources (FHIR) is a crucial step toward solving this problem by providing a standardized API for health data.
The impact of reducing transcription is profound. A 2017 Tufts CSDD study highlighted the rapid planned adoption of eSource, with 87% of research sites finding it helpful. This reflects a clear industry recognition that capturing data at its source is more efficient and accurate, a trend supported by evolving regulations like the US Regulatory Guidance on Using Real-World Data.
Future-Forward: AI, Decentralized Trials, and Federated Platforms
The future of EDC in clinical research is being shaped by three powerful, converging forces that are moving the industry beyond simple data capture toward intelligent data analysis and utilization.
- AI and Machine Learning: AI-driven data cleaning and analytics are revolutionizing data management. AI algorithms can perform ‘intelligent’ data cleaning by identifying complex anomalies and patterns that rule-based edit checks would miss. For example, an AI could flag a patient’s vitals as unusual based on their entire medical history within the trial, not just a single out-of-range value. Natural Language Processing (NLP) can extract structured data from unstructured clinical notes. Predictive analytics can identify sites that are struggling with enrollment or patients who are at high risk of dropping out, allowing for proactive intervention. This shifts the role of the data manager from a reactive data cleaner to a proactive data scientist.
- Decentralized Clinical Trials (DCTs): The COVID-19 pandemic dramatically accelerated the adoption of the Decentralized Clinical Trial Model. Modern EDC systems are the technological backbone of these trials, facilitating remote data capture from ePROs, wearables, and telehealth platforms, and integrating data from home health visits.
- Integration and Federation: The future involves deep integration with diverse Real-World Data sources and the rise of federated platforms for Federated Data Analysis. This represents a paradigm shift from the traditional model of creating a massive, centralized data lake. In a federated model, the sensitive patient data remains securely within its original location (e.g., a hospital’s firewall). The analytical queries are sent to the data, and only the aggregated, non-identifiable results are returned to the central researcher. This approach is the only scalable and secure way to conduct analyses across vast, distributed networks of clinical and real-world data, which is essential for the future of precision medicine and evidence generation.
Frequently Asked Questions about EDC Systems
When diving into EDC in clinical research, many people have similar questions. Here are the most common ones.
How does an EDC system differ from an Electronic Health Record (EHR)?
This is a common question. While both systems handle patient data, they serve different purposes.
An EHR is a patient’s comprehensive medical record, used for ongoing clinical care. It contains a lifelong medical history.
In contrast, an EDC system is a focused research tool for a specific clinical trial. It collects only the data points required by the study protocol to answer a research question safely and effectively.
These systems are beginning to work together through eSource integration, where an EDC can pull relevant data directly from an EHR, reducing manual entry and errors.
Are EDC systems required by regulatory bodies like the FDA or EMA?
Regulatory bodies like the FDA and EMA do not explicitly mandate EDC in clinical research. However, they have strict guidelines for electronic records, such as the FDA’s 21 CFR Part 11, which demand high levels of data integrity.
EDC systems are designed to meet these requirements with features like audit trails and electronic signatures. While not technically required, EDC is the industry standard, and using a compliant system is practically essential for smooth regulatory review and approval.
What are the most common types of data collected in an EDC?
EDC systems are flexible, but most trials collect common data types:
- Patient demographics: Basic information like age and gender.
- Medical history: Past conditions and treatments.
- Vital signs and lab results: Objective measurements like blood pressure and blood tests.
- Adverse events: Tracking any negative reactions for patient safety.
- Concomitant medications: Other drugs a patient is taking.
- Patient-reported outcomes (ePROs): Data on symptoms or quality of life reported directly by patients.
While the specific data varies by study (e.g., tumor measurements in oncology), EDC systems can be customized to capture any required information while maintaining high quality standards.
Conclusion: Powering the Next Generation of Clinical Trials
The evolution of EDC in clinical research is remarkable. From simple digital forms, these systems have become the backbone of modern trials, improving data quality, accelerating timelines, and ensuring compliance. But this is just the beginning. The future lies in integrated ecosystems where EDC connects with EHRs, wearables, and real-world data sources, creating a holistic view of patient health.
The shift is from data capture to intelligent platforms. The future is integrated and federated, enabling analysis across distributed data sources while ensuring privacy and security. As trial complexity and data volumes grow, traditional methods are insufficient. Advanced federated solutions are now essential.
At Lifebit, our next-generation federated AI platform is built for these challenges. It provides secure, real-time access to global biomedical data, with tools for harmonization, AI/ML analytics, and federated governance. Through our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer), we deliver the real-time insights, AI-driven safety surveillance, and secure collaboration that next-generation trials demand. We are committed to making research faster, more compliant, and more impactful for patients.
The journey of EDC in clinical research continues, and we are excited to power that future. Explore how Lifebit’s next-generation platform powers complex clinical research.

