Decoding IRT: The Essential Guide to Interactive Response Technology in Clinical Research
Interactive Response Technology: Decode 2025
Why Interactive Response Technology is the Central Nervous System of Modern Clinical Trials
Interactive response technology (IRT) is a software system that automates and manages critical clinical trial operations including patient randomization, drug supply logistics, and data collection throughout the study lifecycle. As clinical research becomes increasingly complex and globalized, IRT has evolved from a simple randomization tool to the central coordination hub that ensures trial integrity, regulatory compliance, and operational efficiency.
Key Functions of Interactive Response Technology:
- Patient Management: Automated screening, randomization, and blinding protection
- Supply Chain Control: Real-time inventory tracking and automated resupply
- Data Integration: Seamless connection with EDC, CTMS, and other eClinical systems
- Compliance Support: Built-in audit trails and 21 CFR Part 11 validation
- Global Operations: 24/7 availability across multiple sites and time zones
The shift from manual, paper-based trial management to automated IRT systems has transformed clinical research operations. Where sponsors once relied on sealed envelopes for randomization and spreadsheets for supply tracking, modern trials now leverage sophisticated platforms that can build studies in as little as four weeks and manage over 35 million patient transactions across global networks.
This technological evolution addresses critical pain points that have historically plagued clinical trials: human error in randomization, supply chain disruptions, data quality issues, and regulatory compliance challenges. By centralizing these functions within an integrated platform, IRT enables research teams to focus on scientific innovation rather than operational logistics.
As Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit with over 15 years of expertise in computational biology and health-tech entrepreneurship, I’ve witnessed how interactive response technology transforms clinical operations by creating seamless data flows between traditionally siloed systems. My experience building cutting-edge tools for precision medicine has shown me that the future of clinical research depends on integrated platforms that can handle the complexity of modern, data-driven trials.
Key interactive response technology vocabulary:
- clinical research saas technology
- current systems and technology in clinical trials
What is Interactive Response Technology (IRT) and Why is it Crucial?
Think of interactive response technology (IRT) as the mission control center for a clinical trial, coordinating the countless moving pieces of a modern study.
Interactive response technology is essentially a smart software system that takes over the complex job of running clinical trials automatically. Instead of relying on paper forms, phone calls, and manual tracking that can lead to costly mistakes, IRT handles everything digitally with precision and speed.
You might hear IRT called by different names, such as Randomization and Trial Supply Management (RTSM), Interactive Voice Response System (IVRS), or Interactive Web Response System (IWRS). While the acronyms vary, they all refer to the same core concept of a centralized system for trial management.
What makes IRT so powerful is how it connects all the critical pieces of trial management. It automatically assigns patients to treatment groups, tracks drug supplies in real-time, and ensures data flows seamlessly between different systems. This level of automation isn’t just convenient – it’s becoming essential as trials grow more complex and span multiple countries and time zones.
The numbers speak for themselves: industry leaders have managed over 35 million patient transactions across more than 102,000 sites worldwide using these systems. That’s the kind of scale and reliability that transforms how we approach current systems and technology in clinical trials.
The Evolution from IVRS and IWRS to Modern IRT
The story of interactive response technology starts with a simple idea: using phones to make clinical trials more efficient. Early IVRS systems let researchers call in and use their phone’s keypad to randomize patients or check drug supplies. It was for its time, replacing sealed envelopes and manual processes that could introduce errors.
As the internet became widespread, IWRS systems emerged, offering user-friendly web interfaces instead of complex phone menus. This shift greatly improved efficiency and ease of use.
Today’s modern IRT systems, often called IXRS, combine the best of both worlds. They offer web-based convenience as the primary interface while maintaining phone access for situations where internet connectivity might be limited. This integrated approach means research teams can configure new studies in as little as four weeks – a timeline that would have been impossible with older, manual methods.
The shift toward web-based platforms has been particularly dramatic because they’re simply easier to use and more powerful. They can handle complex data interactions, provide real-time dashboards, and support the kind of sophisticated trial designs that modern medicine demands.
Core Purpose in Modern Clinical Research
At its core, interactive response technology exists to solve three fundamental challenges that have plagued clinical research for decades: bias reduction, error minimization, and real-time oversight.
Bias reduction is perhaps the most critical function. In blinded studies, where neither patients nor doctors know who’s receiving the actual treatment versus a placebo, IRT acts like a digital vault. It keeps treatment assignments completely secret while ensuring each patient gets exactly what the study protocol requires. This protection of study integrity is absolutely essential for producing reliable results that can lead to new treatments.
Error minimization happens because computers don’t have bad days, don’t misread handwriting, and don’t accidentally assign the wrong treatment. When human error can compromise patient safety or invalidate years of research, having automated systems manage these critical decisions becomes invaluable.
Real-time oversight means research teams can monitor their trials 24/7, regardless of time zones. Whether it’s a site in Tokyo enrolling a patient at midnight or a clinic in London running low on study drug, the system provides immediate visibility and can trigger automatic responses. This global support capability has become essential as trials increasingly span multiple continents.
Modern IRT systems also excel at supporting complex, adaptive trial designs where treatment assignments might change based on interim results, or where patient eligibility criteria are particularly nuanced. This flexibility allows researchers to tackle more sophisticated questions while maintaining the rigorous standards that regulatory agencies require.
For a deeper dive into how these technologies fit into the broader landscape of clinical research innovation, explore our insights on clinical research technology.
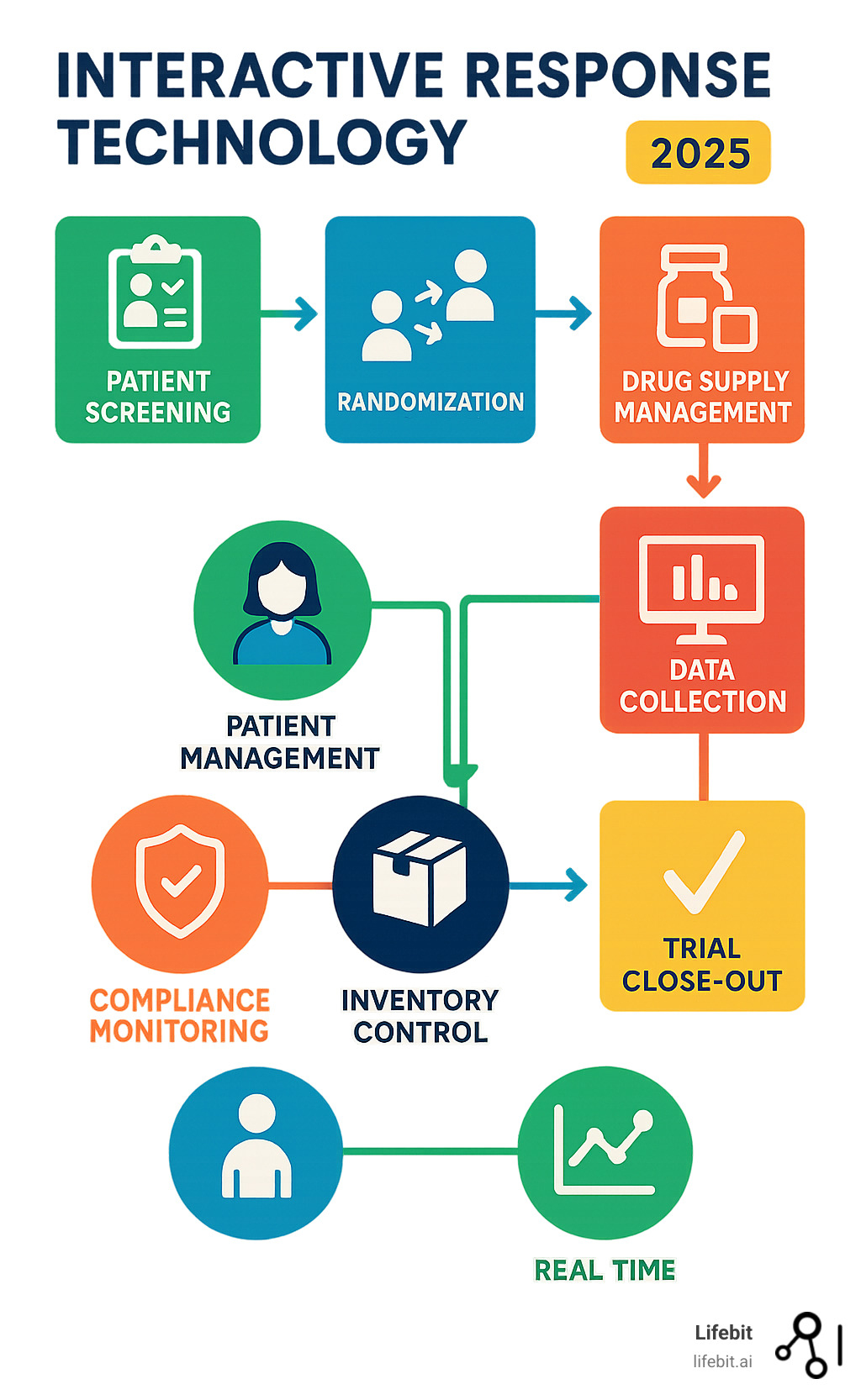
Core Functions of IRT in Clinical Trial Management
Interactive response technology seamlessly connects patient care with supply chain logistics, orchestrating the key elements of a successful clinical trial.
Patient Management: Streamlining Randomization and Blinding
When a patient passes screening, interactive response technology handles one of the most critical moments in clinical research: randomization. This isn’t just about flipping a coin. IRT uses sophisticated methods like central randomization, site-stratified approaches, minimization techniques (such as Pocock-Simon), and even complex adaptive designs to ensure patients are fairly assigned to treatment groups.
The beauty of this system lies in its ability to maintain balance across treatment arms while keeping everyone in the dark. This blinding protection is where IRT truly shines. Through role-based access controls, the system acts like a digital vault, ensuring only authorized personnel can peek behind the curtain when absolutely necessary.
But what happens in a medical emergency? IRT has that covered too. The system can perform emergency unblinding faster than you can say “medical emergency,” immediately notifying the study team and creating an unbreakable audit trail. It can even automatically discontinue a participant if the protocol requires it. For more insights on optimizing patient management in trials, check out our guide on Enhancing Clinical Trial Matching with Lifebit Patient Management.
Drug Supply and Inventory Management (RTSM)
Interactive response technology transforms the precarious balancing act of drug supply management into a well-oiled machine.
The system provides real-time inventory tracking that follows every pill, vial, and dose from the manufacturing depot to the patient’s hands. When supplies start running low, IRT doesn’t wait for someone to notice – it automatically triggers resupply orders based on actual patient demand and predefined thresholds.
Nobody wants to explain to regulators why expired drugs were dispensed to patients. IRT keeps a watchful eye on expiry dates, sending alerts well before products approach their shelf life. It also handles the less glamorous but equally important tasks of drug accountability, managing returns, and coordinating destruction of unused supplies. This level of control means sites always have what they need without drowning in excess inventory.
Data Collection, Reporting, and Compliance
Interactive response technology acts as a data powerhouse. Every action is captured in real-time, creating a complete picture of trial progress.
The system generates comprehensive dashboards that let you monitor everything from enrollment tracking to site performance metrics. No more waiting weeks for status reports – you can see exactly how your trial is performing right now. This real-time visibility means you can spot problems early and make course corrections before they become major headaches.
When it comes to regulatory compliance, IRT doesn’t mess around. Systems are built to meet 21 CFR Part 11 requirements and Good Clinical Practice guidelines from the ground up. Every action creates a secure audit trail that would make any inspector smile. This isn’t just about checking boxes – it’s about building rock-solid evidence that your trial was conducted with integrity. For deeper insights into maintaining data quality throughout your trials, explore our comprehensive guide on Clinical Data Governance.
The difference between manual methods and IRT is like comparing a horse-drawn carriage to a modern car. Manual randomization with sealed envelopes gives way to automated, unbiased assignment. Spreadsheet-based supply management transforms into real-time tracking with predictive resupply. Paper forms evolve into integrated electronic data capture. And delayed, retrospective reporting becomes instant dashboards with actionable insights. The result? Faster trials, better data quality, and regulatory inspectors who actually enjoy their visits.
Key Benefits of Using Interactive Response Technology
The real-world impact of interactive response technology on clinical trials is transformative. IRT fundamentally reshapes how research teams operate, delivering measurable gains in accuracy, speed, cost-effectiveness, and the ability to handle complex studies.
Improving Trial Efficiency and Accuracy
The most striking benefit of interactive response technology is how it eliminates the human errors that plague manual trial management. Think about the traditional approach: sealed envelopes for randomization, spreadsheets for inventory tracking, and phone calls to coordinate supply shipments. Each step introduces opportunities for mistakes that can compromise your entire study.
IRT changes this completely. Automated randomization ensures perfect protocol compliance every time, while real-time inventory management prevents both costly stockouts and wasteful overages. The system works around the clock – literally 24/7 – so enrollment can continue regardless of time zones or office hours.
Here’s what really matters: studies using IRT can be built and deployed in just four weeks, compared to months with traditional methods. Sites report faster patient enrollment because the streamlined processes reduce administrative burden. Research teams can focus on what they do best – the science – rather than wrestling with logistics.
Ensuring Scalability and Flexibility for Complex Trials
Modern clinical research demands incredible flexibility, and interactive response technology delivers exactly that. Whether you’re running a small Phase I study or a massive global Phase III trial spanning multiple continents, IRT scales seamlessly to meet your needs.
The technology excels at managing global multi-site trials with support for dozens of languages across more than 100 countries. But it’s not just about geography – IRT handles the most sophisticated study designs that would be nearly impossible to manage manually. Adaptive trial designs that modify protocols based on interim results, complex randomization schemes using Bayesian methods, and specialized therapies like cell and gene treatments all benefit from IRT’s sophisticated automation.
This flexibility proves invaluable when dealing with emerging trends in clinical research. As regulatory requirements evolve and new therapeutic areas emerge, IRT systems adapt quickly without requiring complete overhauls. For insights into how technology continues to shape study designs, explore Emerging Trends and Technologies in Clinical Trial Design.
Strengthening Regulatory Compliance and Data Integrity
Regulatory compliance isn’t optional in clinical research – it’s the foundation everything else builds upon. Interactive response technology transforms compliance from a constant worry into a built-in advantage through validated systems that meet stringent regulatory standards.
Every IRT system worth considering provides 21 CFR Part 11 compliance, ensuring electronic records and signatures meet FDA requirements. More importantly, the technology creates secure audit trails automatically, documenting every action taken throughout the trial. This comprehensive documentation proves invaluable during regulatory inspections and demonstrates your commitment to Good Clinical Practice (GCP) guidelines.
The data integrity benefits extend beyond just meeting requirements. Real-time data capture eliminates transcription errors, while automated processes ensure consistent application of protocols across all sites. Even established organizations like the American Cancer Society are transitioning from manual methods to purpose-built technology, recognizing how it optimizes time, increases participant engagement, and dramatically improves data quality.
For research teams serious about maintaining regulatory confidence while accelerating their studies, IRT provides the robust foundation needed for success. Learn more about leveraging technology for compliance in our guide on AI for Regulatory Compliance.
The Future of IRT: Integration and Advanced Analytics
The future of interactive response technology lies in breaking down the walls between different clinical systems and using the power of artificial intelligence to make trials smarter and more efficient. We’re moving toward a world where IRT doesn’t just manage trials—it actively helps predict and optimize them.

How to Integrate Interactive Response Technology with Other Systems
Think of modern interactive response technology as the conductor of a clinical trial orchestra. Just as a conductor coordinates different instruments to create beautiful music, IRT orchestrates various eClinical systems to create seamless trial operations.
The magic happens when IRT connects with Electronic Data Capture (EDC) systems. Instead of site staff manually entering the same patient information multiple times, IRT can automatically pull relevant data like patient weight for dosing calculations. This connection eliminates those frustrating double-entry errors that can derail a study.
Clinical Trial Management Systems (CTMS) integration creates a unified view of your entire trial landscape. Site coordinators can see enrollment progress, study milestones, and patient status all in one place. No more switching between multiple systems or wondering if the data matches up.
The integration with Electronic Patient-Reported Outcome (ePRO) and Electronic Clinical Outcome Assessment (eCOA) systems transforms how we capture patient experiences. When a patient completes a quality-of-life questionnaire on their phone, that data flows directly into the trial database, linked to their treatment arm and visit schedule.
For specialized studies, IRT can connect with laboratory and medical imaging systems, ensuring that every test result is properly linked to the right patient and visit. This is particularly valuable for complex oncology or rare disease trials where timing is critical.
These connections work through robust Application Programming Interfaces (APIs) that enable real-time, bidirectional data flow. The result? Reduced data duplication, fewer reconciliation headaches, and improved overall data quality. Our comprehensive guide to Clinical Data Interoperability: A Complete Guide explores how these connections transform clinical operations.
Future Trends and Advancements in Interactive Response Technology
The next generation of interactive response technology is getting a serious upgrade, and artificial intelligence is leading the charge. Instead of simply reordering supplies when inventory runs low, future IRT systems will predict exactly when and how much medication each site will need based on enrollment patterns, seasonal variations, and even local events that might affect patient visits.
Machine learning algorithms will analyze historical data from thousands of trials to forecast supply needs with unprecedented accuracy. Imagine an IRT system that knows a site will need extra medication shipments during flu season because patient visits typically increase, or one that can predict which sites are likely to exceed their enrollment targets based on early performance indicators.
Decentralized Clinical Trials (DCTs) are reshaping how we think about patient participation, and IRT is adapting beautifully. Future systems will seamlessly manage direct-to-patient (DTP) logistics, coordinating medication shipments to patients’ homes while tracking temperature-sensitive biologics through complex cold-chain networks. They’ll integrate data from wearable devices, smartphone apps, and home monitoring equipment, creating a complete picture of patient engagement and outcomes.
The user experience is getting a major makeover too. Tomorrow’s IRT systems will feature intuitive interfaces that work just as well on a smartphone as they do on a desktop computer. Site coordinators will be able to randomize patients, check inventory, and generate reports from anywhere, making trials more flexible and responsive.
Perhaps most exciting is the integration with Real-World Data (RWD) sources. By combining IRT data with electronic health records, insurance claims, and other real-world evidence, we’ll gain deeper insights into how treatments perform outside the controlled trial environment. This connection will support more sophisticated adaptive trial designs and provide valuable post-market surveillance data. Learn more about how we leverage Real-World Data in Clinical Trials.
Blockchain technology is also entering the picture, offering an immutable record of every drug shipment and patient interaction. This creates an unbreakable chain of custody that regulators love and sponsors can trust completely.
These advances position interactive response technology as more than just a trial management tool—it’s becoming the intelligent backbone of modern, patient-centric clinical research. The future promises trials that are more efficient, more accurate, and ultimately more successful at bringing life-changing treatments to patients who need them. Find how AI for Clinical Trials is changing every aspect of clinical research.
Frequently Asked Questions about Interactive Response Technology
What is the difference between IRT and RTSM?
Interactive response technology (IRT) serves as the umbrella term for all systems that automate patient interactions and trial logistics. Think of it as the broader family name that includes various types of clinical trial management systems.
RTSM, which stands for Randomization and Trial Supply Management, is actually a more modern and precise way to describe what these systems do. While IRT might sound a bit technical and broad, RTSM gets straight to the point – it tells you exactly what the system handles: randomizing patients and managing your drug supply.
The truth is, these terms are often used interchangeably in today’s clinical research world. However, there’s a subtle difference worth noting. Older interactive response technology systems might have focused primarily on basic voice or web interactions. Modern RTSM platforms, on the other hand, emphasize the complete integration of patient randomization with sophisticated supply chain management.
All RTSM systems are technically a type of IRT, but not every older IRT system offers the full range of capabilities you’d expect from today’s comprehensive RTSM solutions.
How does IRT support decentralized clinical trials (DCTs)?
Decentralized Clinical Trials represent a fundamental shift in how we conduct research, and interactive response technology has become absolutely essential for making them work smoothly. When patients participate from their homes rather than visiting clinical sites, the logistics become significantly more complex.
IRT handles the intricate process of shipping investigational products directly to patients’ homes, which we call direct-to-patient (DTP) logistics. This isn’t just about mailing a package – the system needs to track temperature-sensitive medications, manage delivery schedules, and ensure patients receive the right treatment at the right time.
The technology also excels at coordinating remote patient visits and integrating data from various digital health tools like wearables, mobile apps, and home monitoring devices. This creates a seamless flow of information that keeps the trial coordinated and compliant, even when participants are scattered across different locations.
What makes interactive response technology particularly valuable for DCTs is its ability to bridge the gap between traditional site-based trials and patient-centric remote models. It maintains the same level of oversight and data quality while adapting to this new, more flexible approach to clinical research. For comprehensive insights into this evolving field, explore our guide on Decentralized Clinical Trials: Guidance.
Is an IRT system required for every clinical trial?
While regulatory agencies don’t mandate interactive response technology for every single clinical trial, it has become essential for the vast majority of modern studies. The question isn’t really whether you need it, but rather whether you can afford to conduct your trial without it.
For very small, single-site, open-label studies, you might technically manage with manual methods like sealed envelopes and spreadsheets. However, even these smaller studies benefit significantly from IRT’s accuracy, built-in compliance features, and audit trail capabilities.
As soon as your trial involves multiple sites, blinding, or complex randomization schemes, interactive response technology becomes practically indispensable. The system’s advantages in reducing human error, maintaining study integrity, optimizing drug supply, and ensuring regulatory compliance far outweigh any initial setup considerations.
The reality is that clinical trials have become increasingly sophisticated, and the manual methods of the past simply can’t keep pace with today’s regulatory requirements and operational demands. Most research organizations find that robust IRT solutions not only improve their current studies but also position them for success as their research programs grow and evolve.
Conclusion
As we’ve explored throughout this guide, interactive response technology serves as the central pillar that supports modern clinical trials. It’s remarkable how this sophisticated system has evolved from simple voice-based randomization to become the operational backbone that enables groundbreaking medical research.
Think about it: every successful clinical trial today relies on IRT to carefully manage patient randomization while protecting study blinds, orchestrate complex drug supply chains across global networks, and ensure unwavering regulatory compliance. This isn’t just automation for automation’s sake – it’s about fundamentally changing how clinical research operates, leading to unprecedented efficiency, superior data quality, and rock-solid reliability.
The change has been profound. Where researchers once struggled with sealed envelopes, spreadsheets, and manual processes prone to human error, we now have integrated platforms that can build studies in four weeks and manage millions of patient transactions seamlessly. This shift has freed research teams to focus on what truly matters: scientific innovation and bringing life-changing therapies to patients.
Looking ahead, the future of clinical research is becoming increasingly integrated and data-driven. At Lifebit, we recognize that open uping the full potential of this future requires secure, real-time access to vast and diverse biomedical data. Our next-generation federated AI platform perfectly complements the operational strengths of interactive response technology by enabling secure, compliant access to global biomedical and multi-omic data.
Our platform’s components work together seamlessly: the Trusted Research Environment (TRE) ensures secure collaboration, the Trusted Data Lakehouse (TDL) manages complex data ecosystems, and R.E.A.L. (Real-time Evidence & Analytics Layer) delivers instant insights and AI-driven safety surveillance. With built-in capabilities for harmonization, advanced AI/ML analytics, and federated governance, we power large-scale research across biopharma, governments, and public health agencies.
This integration matters because the rich data generated by IRT systems can be fully leveraged for scientific findy when combined with our cutting-edge data platforms. We believe this powerful combination of operational excellence from interactive response technology and advanced data analytics can dramatically accelerate the pace of scientific findy.
The goal remains unchanged: bringing life-changing therapies to patients faster, more safely, and with greater precision than ever before.

