Interactive Voice/Web Response Systems: A Deep Dive for Clinical Research
Interactive voice response system clinical trials: Top 1
Why Interactive Voice Response Systems Are Essential for Modern Clinical Trials
Interactive voice response system clinical trials have revolutionized how researchers manage patient enrollment, randomization, and drug supply in global studies. These automated systems allow participants and site staff to interact with centralized databases via phone or web, streamlining trial operations that once relied on manual processes prone to error.
Key components of interactive voice response systems in clinical trials:
- Patient Management: Automated enrollment, eligibility verification, and randomization
- Drug Supply Management: Real-time inventory tracking and automated resupply
- Data Collection: 24/7 access to centralized trial databases
- Regulatory Compliance: Built-in audit trails and GCP adherence
- Global Accessibility: Phone and web-based access from any location
As one industry expert noted, “IxRS service providers were initially ‘dreaded’ by other technological innovations like EDC systems, but have seen rapid growth in use” due to their proven ability to reduce manual errors and improve trial efficiency. Modern systems evolved from simple phone interactions to sophisticated web platforms that integrate with electronic data capture (EDC) and clinical trial management systems (CTMS).
The shift from traditional Interactive Voice Response Systems (IVRS) to Interactive Web Response Systems (IWRS) reflects the broader digitization of clinical research. While IVRS relied on telephone keypad inputs, IWRS leverage web browsers to provide richer data collection capabilities and real-time integration with other trial technologies.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. I’ve spent over 15 years developing platforms that transform healthcare through federated data analysis, including solutions that improve interactive voice response system clinical trials in secure, compliant data environments. My experience has shown me how critical integrated data systems are for advancing precision medicine and streamlining clinical operations.
Interactive voice response system clinical trials terms you need:
- clinical research saas technology
- current systems and technology in clinical trials
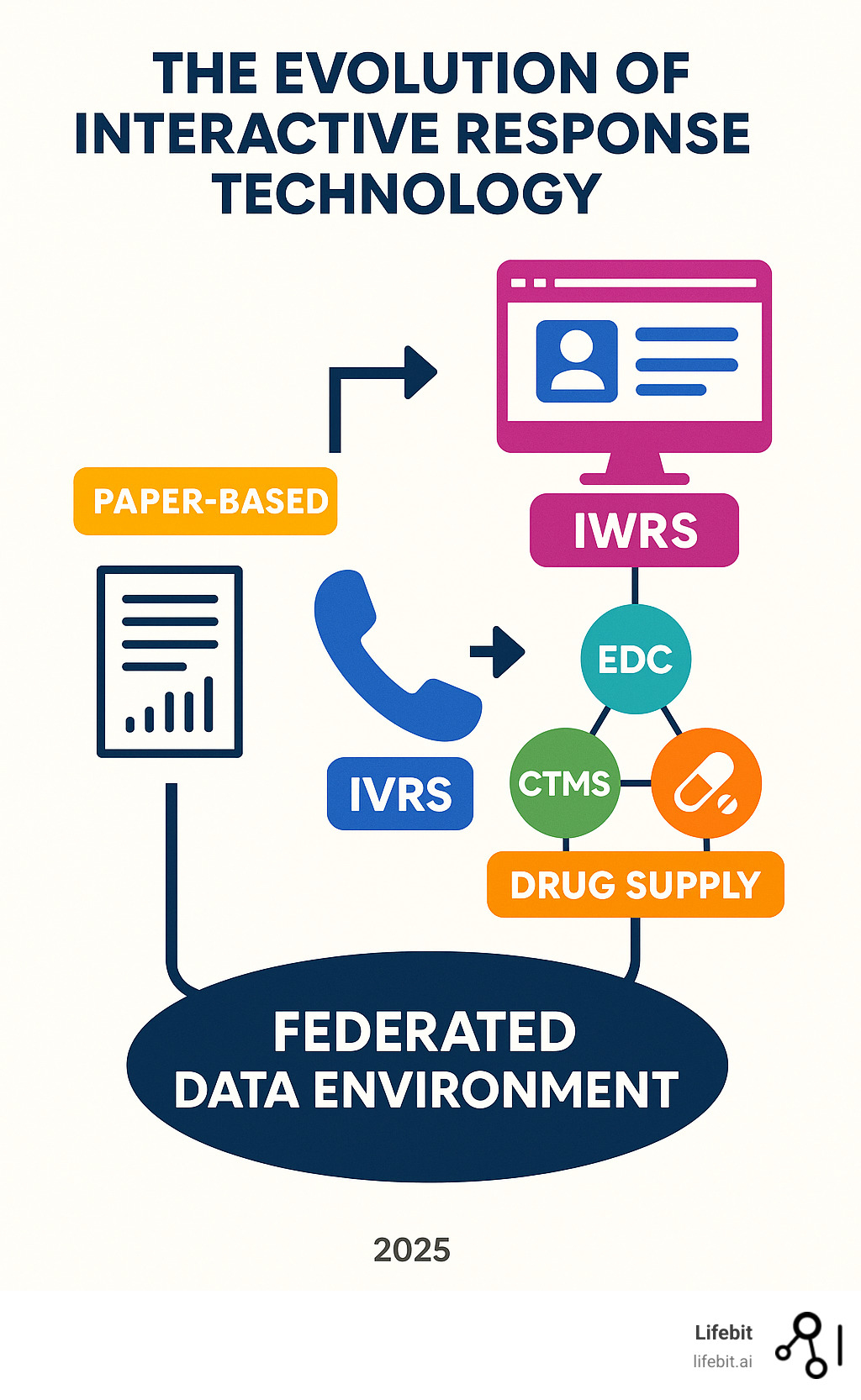
The Evolution of Interactive Response Technology (IRT)
Clinical trials have evolved from manual, paper-based processes into sophisticated digital ecosystems. At the center of this change is Interactive Response Technology (IRT) – the digital backbone of modern trials.
Defining the Core Technologies: From IVRS to IWRS
Early Interactive Voice Response Systems (IVRS) brought automation to clinical trials through telephone keypads, similar to a bank’s automated phone menu. An IVRS allowed researchers and patients to interact with trial databases using a phone, enabling a coordinator to randomize a patient at any time. This was when internet access was unreliable, giving trials 24/7 accessibility and removing geographical barriers for global studies.
As the internet became ubiquitous, Interactive Web Response Systems (IWRS) emerged. Users could now use web interfaces on their computers, making interactions faster and more powerful. IWRS enabled rich visual displays, complex data entry, and real-time integration with other trial systems. While the term interactive voice response system clinical trials is still used, most sites now rely on web-based platforms.
| Feature | Interactive Voice Response System (IVRS) | Interactive Web Response System (IWRS) |
|---|---|---|
| Access Method | Telephone (landline or mobile) | Web browser (computer, tablet, smartphone) |
| User Interface | Voice prompts, keypad input (DTMF tones) | Graphical User Interface (GUI), clickable elements, text input |
| Integration | Limited, often via batch transfers or basic data feeds | Extensive, real-time integration with other eClinical systems (EDC, CTMS) |
| Data Richness | Simpler data points, primarily numerical or short text | Richer data types, complex forms, visual data representation |
| Typical Use Case | Patient reminders, simple data capture, accessibility in low-internet areas | Primary system for randomization, drug supply, complex data management |
Understanding IRT, IxRS, and RTSM
The terminology can be confusing. Interactive Response Technology (IRT) is the umbrella term for all these interactive systems.
IxRS (Interactive eXtended Response System) is a hybrid, offering both voice and web capabilities for maximum flexibility. Randomization and Trial Supply Management (RTSM) describes what these systems do: assign patients to treatment groups and manage drug logistics.
In practice, IRT and RTSM are often used interchangeably. IRT is the technology platform, while RTSM describes the essential trial management processes it enables.
Modern hybrid systems have blurred these lines. Today’s IRT platforms are sophisticated, cloud-based solutions that can switch between voice and web interfaces, designed to handle the complexity of global trials while remaining simple for site staff to use.
The beauty of this evolution is that it has given researchers unprecedented control over their studies while making the technology more accessible than ever. Modern IRT systems provide the scalability and reliability that today’s clinical research demands.
Core Functions and Benefits of an interactive voice response system clinical trials
IRT systems transform the daily chaos of managing complex studies. For a global trial with multiple sites, hundreds of patients, and thousands of drug kits, IRT acts as a digital trial manager, handling the heavy lifting so you can focus on patients.
Streamlining Patient Management and Randomization
Patient management is the foundation of every successful trial. IRT systems choreograph the complex process of eligibility checks, randomization, and visit scheduling, all while maintaining the blind.
Patient enrollment and eligibility verification become automatic safety nets. The IRT system guides staff through inclusion and exclusion criteria, preventing ineligible enrollments, protocol deviations, and wasted time.
The automated randomization feature is a core strength. IRT handles simple or complex stratified randomization with mathematical precision, ensuring balance across patient groups while maintaining the blind.
Maintaining the blind is crucial for scientific integrity. IRT systems securely manage treatment assignments. If emergency unblinding is necessary, the system provides controlled, documented access while maintaining the study’s overall integrity.
Visit scheduling and patient reminders transform IRT into a patient-facing helper. The system can automatically call or email patients about upcoming visits, reducing no-shows and keeping studies on track.
Optimizing Drug Supply and Inventory Management
Drug supply management is the circulatory system of clinical trials. IRT systems excel at this complex logistics puzzle.
Investigational product tracking provides real-time visibility into every drug kit in the supply chain, from a central depot to the patient.
Automated re-supply algorithms act like smart shopping assistants for trial sites. They monitor dispensing patterns, predict needs, and trigger shipments before shortages occur.
The beauty of inventory optimization is its ability to balance priorities. IRT systems track expiry dates and manage complex dosing schedules to ensure enough supply without creating excessive waste.
When serious adverse events require emergency unblinding, IRT provides controlled access with detailed audit trails. The system also handles end-of-study product reconciliation for regulatory compliance.
For a deeper dive into how these systems work, this comprehensive overview of IRT in clinical research provides excellent technical details.
Key Advantages: Efficiency, Accuracy, and Compliance
The improvements from interactive voice response system clinical trials create a ripple effect across all study conduct.
Reduced manual errors is a benefit. IRT systems eliminate human error in repetitive tasks like randomization and inventory tracking, ensuring accuracy in treatment assignments and drug shipments.
Real-time data access enables proactive decision-making. Sponsors can monitor trials continuously, allowing problems to be solved before they derail study timelines.
Cost savings are significant. Reduced drug waste, faster enrollment, and fewer protocol deviations lead to a compelling return on investment.
Scalability for global trials is seamless. IRT systems handle multiple time zones, languages, and regulatory requirements, whether for 10 patients or 10,000.
Improved regulatory compliance is essential. IRT systems are built with Good Clinical Practice (GCP) and 21 CFR Part 11 requirements in mind. Every action creates an audit trail, and every process follows validated procedures. The FDA guidance on computerized systems in clinical investigations provides the framework that reputable IRT systems follow.
Integration, Best Practices, and the Future of IRT
Successfully implementing interactive voice response system clinical trials means weaving the technology into your entire clinical research ecosystem. When done right, IRT becomes the heart of your trial operations.
Best Practices for Integrating IRT into Your Trial
Integrating an IRT system requires careful planning and execution.
Strategic planning is the foundation. Before evaluating vendors, map out your study’s unique needs, such as randomization requirements and supply chain complexity. This ensures your IRT solution is a perfect fit.
Vendor selection goes beyond feature lists. Look for partners with experience in your therapeutic area and similar study designs. The best vendors are collaborators who offer insights, robust integration, and strong support.
User training is critical. Site staff and investigators must be confident using the system from day one. Comprehensive training ensures the technology is used correctly and helps users understand how their actions impact the trial.
System validation ensures patient safety and data integrity. IRT systems undergo rigorous testing to meet standards like GAMP4 and ISO9000:2000. This process gives you confidence that everything will function correctly when real patients are involved.
The magic happens when your IRT system integrates seamlessly with other eClinical platforms. It can orchestrate data flow between your Electronic Data Capture (EDC) system, Clinical Trial Management System (CTMS), and Electronic Patient-Reported Outcomes (ePRO) systems, eliminating duplicate data entry and reducing errors.
Future Trends for interactive voice response system clinical trials
The future of interactive voice response system clinical trials is exciting, with advances that once seemed like science fiction.
Artificial intelligence and machine learning are changing IRT. These technologies enable predictive supply forecasting, which optimizes inventory levels to reduce waste and stockouts. They can also help predict patient dropout rates and identify potential protocol deviations.
Direct-to-patient models are reshaping clinical trials. IRT systems are evolving to manage the logistics of shipping drugs to patients’ homes, including tracking shipments and ensuring drug accountability.
Mobile health and wearable integration will allow for continuous patient monitoring. Future IRT systems will connect with devices to collect real-time health data, creating a richer picture of patient wellbeing.
Increased patient-centricity means IRT systems are becoming more intuitive and personalized, offering custom communication and intelligent reminders.
Adaptive trial designs represent an exciting frontier. These innovative study designs allow researchers to modify trials based on accumulating data. IRT systems provide the real-time data flow and sophisticated randomization algorithms that make these dynamic approaches possible.
Frequently Asked Questions about IRT in Clinical Trials
Here are answers to the most common questions about implementing interactive voice response system clinical trials.
What is the difference between IRT and RTSM?
The relationship between these terms is straightforward.
IRT (Interactive Response Technology) is the technology platform—the software engine that allows users to interact with trial databases via phone or web.
RTSM (Randomization and Trial Supply Management) describes what the technology does: the core functions of assigning patients to treatments and managing drug supply logistics.
In practice, the terms are often used interchangeably. An IRT system is the technology that delivers RTSM functions.
How long does it take to set up an IRT system?
Setup times have shrunk dramatically. Historically, setting up an IVRS took months, requiring massive specification documents and custom programming.
Modern IRT platforms are highly configurable, not custom-coded. This reduces setup times from months to weeks. The primary factor affecting the timeline is study complexity. A simple study might take a few weeks, while a complex adaptive trial will take longer. Involving your IRT provider early in protocol development is key to establishing realistic timelines.
Is an interactive voice response system clinical trials still relevant today?
Absolutely, though its role has evolved. While web-based systems (IWRS) are now standard, phone-based IVRS remains important.
Accessibility is crucial in areas with poor internet. A simple phone call can include populations that would otherwise be excluded.
Patient engagement benefits from IVRS, as automated phone reminders can be highly effective, especially for patients who prefer voice communication.
Emergency situations also benefit from the reliability of a phone-based backup for critical functions like unblinding.
Modern IRT platforms often combine both technologies, using web interfaces for daily operations and phone systems for reminders and backup, ensuring research remains accessible and robust.
Conclusion: Driving the Future of Clinical Research
IRT solutions have evolved from simple phone systems to become the backbone of modern clinical research, making trials more efficient, accurate, and patient-friendly. These platforms are the unsung heroes of clinical research, enhancing data integrity by eliminating the manual errors of paper-based trials.
The impact on patient safety is significant. Controlled randomization, drug tracking, and secure unblinding capabilities protect trial participants by ensuring the right patient gets the right treatment at the right time.
Operationally, the efficiency gains are remarkable. Real-time data access and automated supply chain management prevent costly delays. Achieving operational efficiency through IRT translates directly into faster trial completion and lower costs, helping bring treatments to patients sooner.
Looking ahead, the future of clinical research involves AI-driven logistics, direct-to-patient models, and wearable data integration. Interactive voice response system clinical trials will continue to evolve to support these innovations.
The true potential emerges when IRT systems integrate into a broader ecosystem of federated data and AI. At Lifebit, we see the power of securely connecting IRT data with real-world evidence and genomic datasets. Our platform enables these connections safely and compliantly, allowing researchers to find answers to previously unanswerable questions. This comprehensive analysis can reveal insights that accelerate drug findy and help us understand which treatments work best for which patients.
The future of clinical research is an interconnected ecosystem where data flows securely and insights emerge naturally. That’s the vision we’re building toward, one federated analysis at a time.
Find how a unified data platform can accelerate your research.



