The Clinical Trial Journey Explained
Clinical Trial: 4 Phases, Ultimate Guide
Clinical Trials: Your Gateway to Tomorrow’s Cures
A clinical trial is a research study where new medical treatments are tested on human volunteers to prove their safety and effectiveness. These studies are the final, critical step before a new therapy—from antibiotics to cancer immunotherapies—can reach patients worldwide. Without them, medical progress would stop.
While the process can seem complex, clinical trials follow a structured, highly regulated path designed to protect participants while advancing science. Understanding this journey is key for patients, caregivers, and anyone curious about how new medicines are made.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in computational biology, I’ve seen how clinical trials generate the evidence that transforms global healthcare. At Lifebit, we build secure, federated platforms that help researchers accelerate clinical trial data analysis, bringing new treatments to patients faster.
Terms related to clinical trial:
What Is a Clinical Trial? How Science Vets New Treatments
A clinical trial is a research study designed to answer one fundamental question: “Is this new medical intervention safe and does it work?” It’s the final, rigorous test for everything from new drugs and medical devices to surgical techniques and behavioral therapies before they can be considered for widespread use.
According to the NIH’s definition of a clinical trial, it is a study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. This definition serves as a global standard for medical research.

The Many Purposes of Clinical Trials
The core purpose of a clinical trial is to generate high-quality evidence to improve human health. However, they are not a monolith; different types of trials answer different questions:
- Treatment Trials: The most common type, these studies test new treatments (like a new chemotherapy drug), new combinations of drugs, or new approaches to surgery or radiation therapy.
- Prevention Trials: These look for better ways to prevent diseases in people who have never had them or to prevent a disease from returning. This can include medicines, vaccines, vitamins, or lifestyle changes.
- Diagnostic Trials: These are conducted to find better tests or procedures for diagnosing a particular disease or condition. For example, a new imaging technique or a less invasive biopsy method.
- Screening Trials: These test the best way to detect certain diseases or health conditions at an early stage, often before symptoms appear.
- Quality of Life Trials (Supportive Care): These explore ways to improve comfort and the quality of life for individuals with chronic illnesses. They might evaluate interventions that manage symptoms and side effects from treatment, such as nausea or pain.
The Ethical Foundation of Modern Research
Modern clinical trials are built on a bedrock of strict ethical principles designed to protect participants. This was not always the case. Abuses in the 20th century led to the creation of foundational ethical codes. The Nuremberg Code (1947), established after WWII, was the first to mandate voluntary consent. This was followed by the Declaration of Helsinki (1964), which further elaborated on the ethical responsibilities of physicians in research. In the U.S., the Belmont Report (1979) established three core principles:
- Respect for Persons: Protecting the autonomy of all people and treating them with courtesy and respect, which includes the necessity of informed consent.
- Beneficence: The philosophy of “do no harm” while maximizing benefits for the research project and minimizing risks to the research subjects.
- Justice: Ensuring reasonable, non-exploitative, and well-considered procedures are administered fairly and that the distribution of costs and benefits is equitable.
These principles are the pillars that support every aspect of a modern clinical trial, from its design to its execution.
How is a clinical trial different from standard treatment?
While both aim to improve health, they operate differently. Standard treatment uses proven therapies customized to you as an individual by your doctor. A clinical trial is a research study designed to gather data on an experimental intervention according to a strict scientific plan.
Key differences include:
- Fixed Protocol: Trials follow a rigid plan, or protocol, that specifies exactly what will be done and when. This plan cannot be altered for individuals, ensuring the data collected is consistent and scientifically reliable.
- Experimental Intervention: You may receive a treatment whose full range of effects, both positive and negative, are not yet fully understood. This is the central question the trial aims to answer.
- Intensive Data Collection: Trials typically involve more frequent tests, scans, and monitoring than standard care. This is necessary to meticulously track the intervention’s effects and ensure participant safety.
- Control Group: To understand if the new treatment is effective, it must be compared to something else. You might be randomly assigned to a control group that receives a placebo (an inactive substance) or the current standard-of-care treatment.
Throughout the trial, your regular healthcare team continues to manage your overall health, while the specialized trial team focuses on administering the experimental treatment and collecting data related to the study.
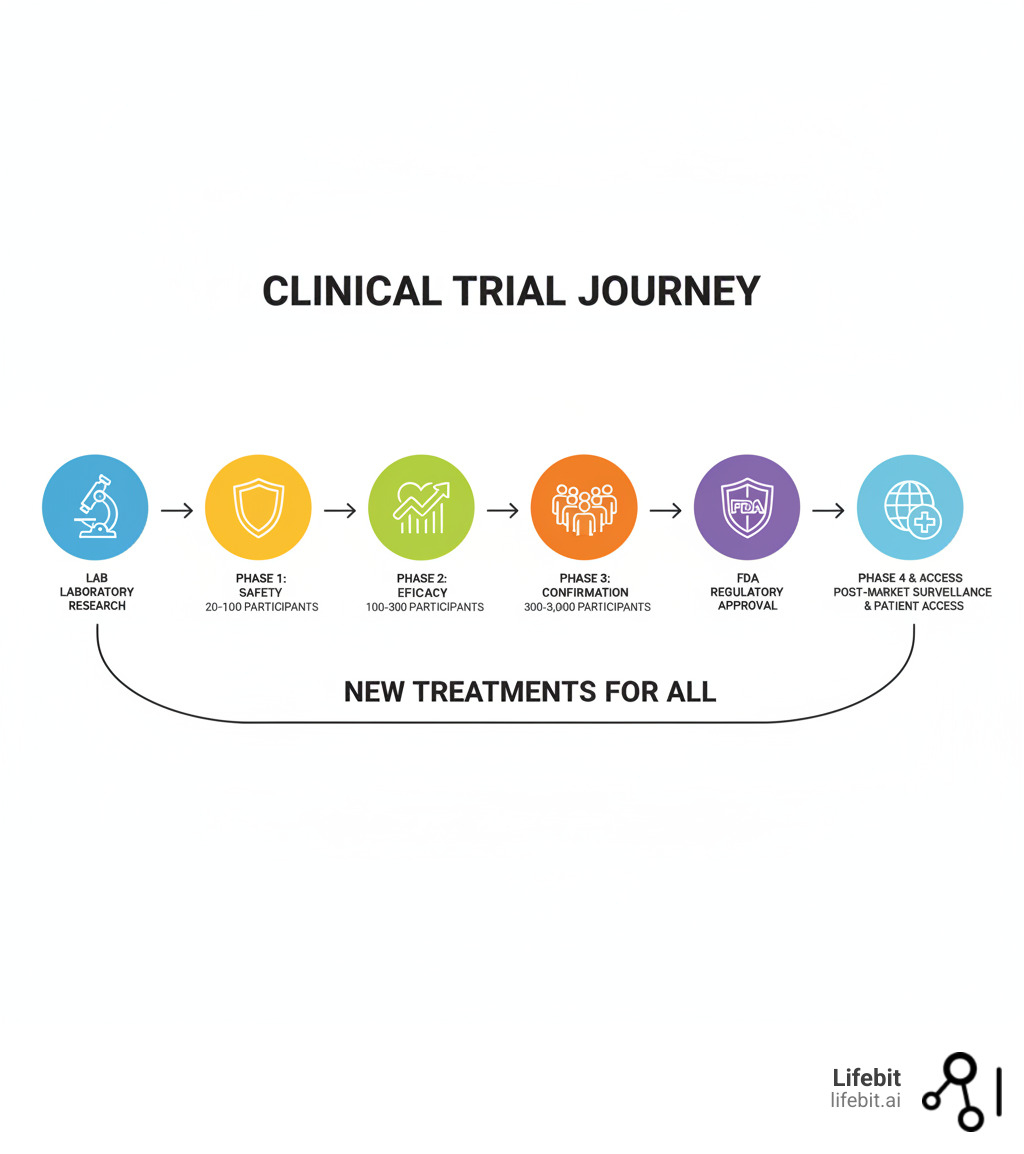
The Phases of a Clinical Trial: A Step-by-Step Journey
The journey from a promising idea in a lab to a medicine in your local pharmacy is a long, expensive, and meticulously regulated process. This journey is divided into distinct clinical trial phases, each with a specific purpose. This systematic approach is designed to build a comprehensive safety and efficacy profile, protecting participants while ensuring only beneficial treatments reach the public.
Before the Trial Begins: The Pre-clinical Stage
Before any testing in humans, a potential new drug undergoes extensive pre-clinical testing. This involves laboratory research (in vitro studies) to understand its basic properties and animal studies (in vivo studies) to evaluate its safety and potential efficacy. Researchers gather data on toxicity and how the drug is absorbed, distributed, metabolized, and excreted (ADME). Only if a compound shows promise and an acceptable safety profile in this stage can a sponsor apply to a regulatory body like the FDA to begin testing in humans.
Phase 0: Exploratory First-in-Human Studies
Sometimes, a very small, optional first step called a Phase 0 trial is conducted. These are not designed to test safety or efficacy.
- Participants: A very small number of people (fewer than 15).
- Goal: To see if the drug behaves in humans as expected from pre-clinical studies. Participants are given a single, sub-therapeutic microdose—a dose too small to cause any therapeutic effect or harm. Advanced imaging or biopsy techniques are then used to confirm that the drug reaches its target tissue and to study its basic pharmacokinetics. This can help weed out unpromising candidates early, saving time and money.
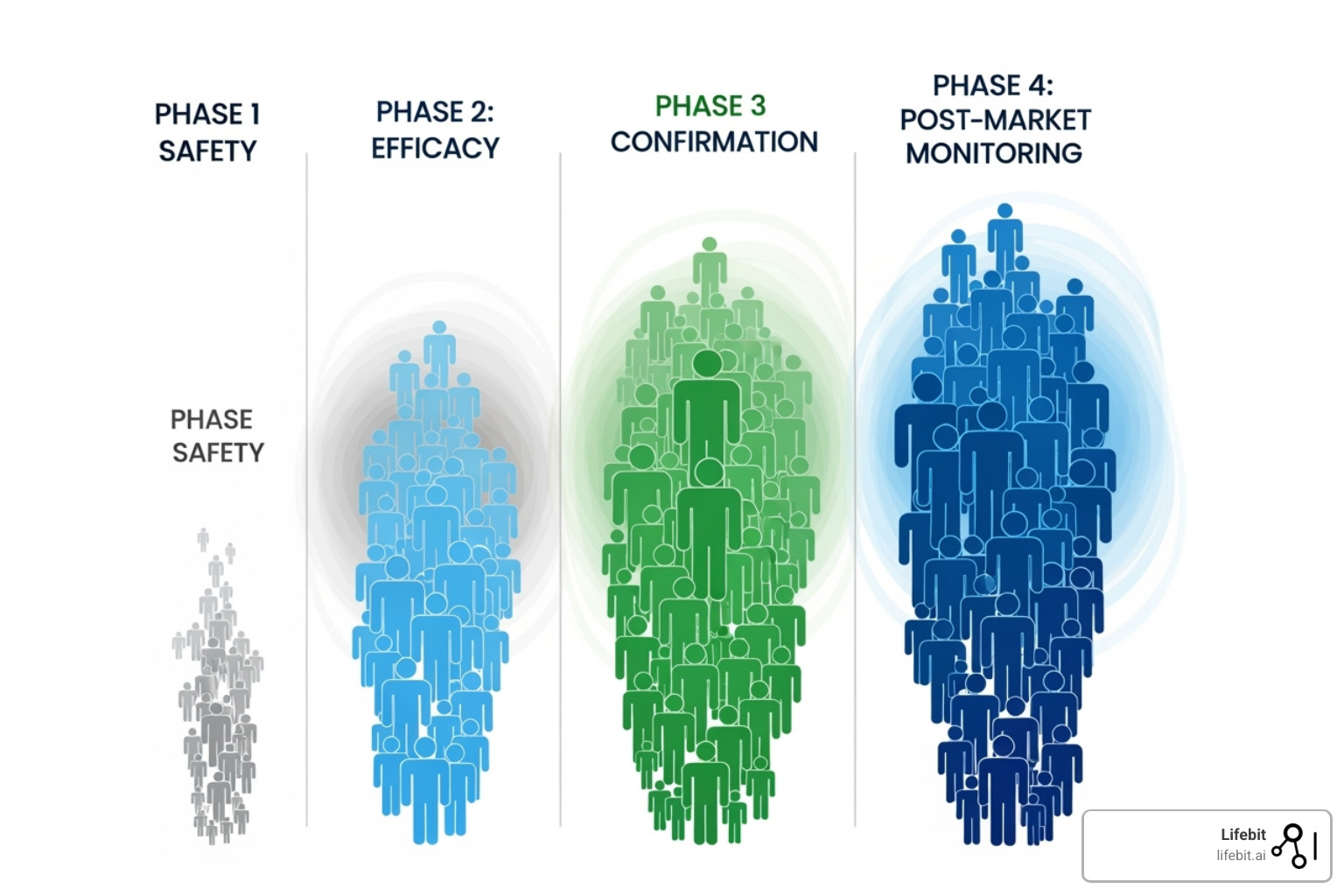
Phase 1: Is It Safe for Humans?
This is the first time the investigational drug is tested in a small group of people to evaluate its safety.
- Participants: A small group of 20 to 100 healthy volunteers, or in some cases (like oncology), patients with the target disease.
- Goal: The primary goal is to determine a safe dosage range and identify side effects. Researchers conduct dose-escalation studies, such as Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) studies, to find the maximum tolerated dose (MTD). This phase also involves intensive monitoring of pharmacokinetics (what the body does to the drug) and pharmacodynamics (what the drug does to the body).
- Duration: Several months.
Phase 2: Does It Work and What’s the Right Dose?
Once a drug is deemed safe in Phase 1, the focus shifts to its effectiveness in patients with the condition it’s meant to treat.
- Participants: A larger group of 100 to 300 patients.
- Goal: This phase is often split into two parts. Phase 2a is a “proof-of-concept” study to see if the drug has any biological activity or effect on the disease. Phase 2b is a “dose-ranging” study to determine the optimal dose that provides the best efficacy with the fewest side effects. For the first time, patients may be randomized into different groups, with some receiving the investigational drug and others receiving a placebo or the standard treatment.
- Duration: Several months to two years.
Phase 3: Is It Better Than What We Have Now?
This is the large-scale, pivotal phase designed to provide the definitive evidence needed for regulatory approval.
- Participants: A large, diverse group of 300 to 3,000+ patients, often recruited from multiple clinics and countries to ensure the results are generalizable.
- Goal: To confirm the drug’s effectiveness, monitor side effects, and compare it to the current standard of care or a placebo. These are almost always randomized, double-blind trials, meaning neither the participant nor the investigator knows who is receiving the experimental drug versus the control. The trial must meet pre-specified primary endpoints (e.g., improved survival, reduced tumor size) with statistical significance to be considered a success. The robust data from Phase 3 trials forms the core of the New Drug Application (NDA) submitted to the FDA.
- Duration: One to four years or more.
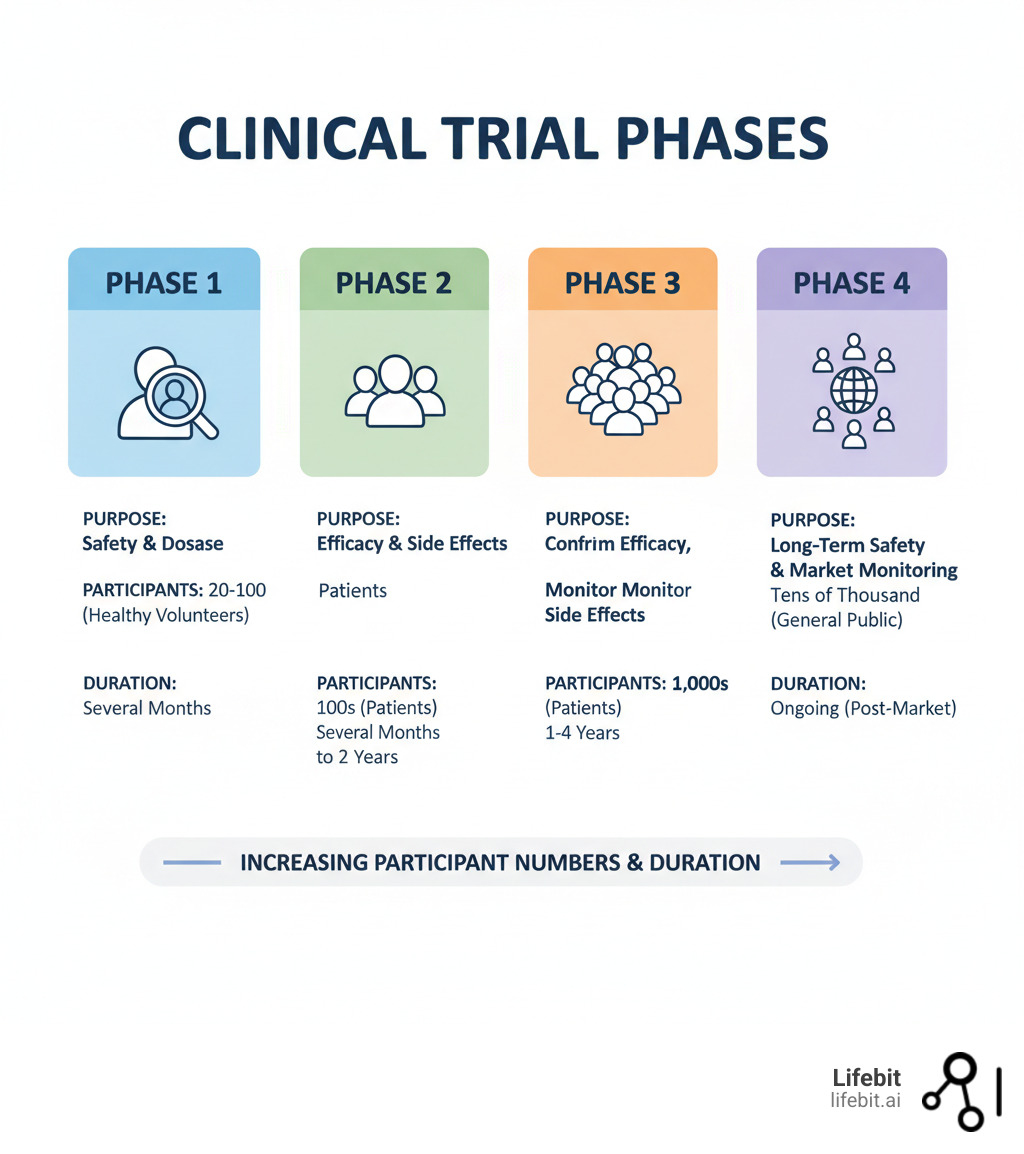
Phase 4: What Happens in the Real World?
After a treatment is approved and on the market, monitoring continues in what are known as post-market surveillance studies.
- Participants: Thousands of patients receiving the treatment as part of their regular medical care.
- Goal: To track the drug’s long-term safety and effectiveness in a broad, real-world population. This process, called pharmacovigilance, can uncover rare or long-term side effects not seen in the more controlled environment of Phases 1-3. Phase 4 studies can also explore new uses for the drug or how it performs in specific subgroups of the population, generating valuable Real-World Evidence (RWE).
- Duration: Ongoing for as long as the treatment is available.
The Unseen Safety Net: How Trials Are Designed to Protect You
Before a single participant is enrolled, a clinical trial undergoes months or even years of meticulous planning and independent review. This rigorous setup process is a critical safety system designed to protect participants, uphold ethical standards, and ensure the scientific validity of the results.
Developing the Study Protocol: The Master Blueprint
Every trial is guided by a protocol, a detailed master plan that acts as the study’s rulebook. This document, often hundreds of pages long, is developed based on prior lab and animal studies and outlines every aspect of the study with painstaking precision. Its key components include:
- Rationale and Objectives: A clear explanation of the scientific background and the specific questions the trial aims to answer.
- Eligibility Criteria: A detailed list of inclusion and exclusion criteria defining exactly who can and cannot participate in the study.
- Study Design and Procedures: A full timeline of all tests, procedures, medication schedules, and participant visits.
- Dosages and Administration: Precise instructions on how the investigational treatment is to be prepared and administered.
- Endpoints: The specific, measurable outcomes that will be used to determine if the treatment is safe and effective (e.g., change in a biomarker, tumor response rate, overall survival).
- Statistical Analysis Plan: A pre-specified plan for how the data will be collected, managed, and analyzed to prevent bias in interpretation.
- Safety Monitoring Plan: A detailed strategy for identifying, evaluating, and reporting adverse events.
Who Ensures Participant Safety? A Multi-Layered System
Multiple independent bodies oversee clinical trials to ensure they are conducted ethically and safely. This creates a system of checks and balances.
- Institutional Review Boards (IRBs): An IRB (or Ethics Committee) is a committee of physicians, scientists, statisticians, and community members at each research institution. Their primary responsibility is to protect the rights and welfare of human research subjects. They must review and approve the study protocol, informed consent form, and all participant-facing materials before the trial can begin. They continue to monitor the study’s progress at least annually.
- Food and Drug Administration (FDA): In the U.S., the FDA plays a critical oversight role. For new drugs, sponsors must submit an Investigational New Drug (IND) application, which the FDA reviews for safety before allowing human trials to start. The FDA can place a trial on “clinical hold” at any time if safety concerns arise.
- Data and Safety Monitoring Boards (DSMBs): For larger, randomized trials (especially Phase 3), an independent group of experts, including clinicians and biostatisticians, forms a DSMB. This board is the only group that periodically reviews the unblinded trial data as it accumulates. They watch for safety issues and can recommend that a trial be modified or stopped early, either due to safety concerns or if the treatment shows overwhelming benefit.
- International Regulations: In Europe, the European Medicines Agency (EMA) performs a similar function to the FDA. Global standards are often guided by the International Council for Harmonisation (ICH) Good Clinical Practice (GCP) guidelines, which provide a unified standard for the design, conduct, and reporting of clinical trials to ensure data is credible and that subjects’ rights are protected.
The Informed Consent Process: Your Right to Know
Informed consent is not just a signature on a form; it is a fundamental right and an ongoing process of communication. Before agreeing to participate, you will have a detailed conversation with the research team and receive a written document that explains the study in clear, non-technical language. This document must explain:
- The study’s purpose, duration, and procedures.
- That the intervention is experimental.
- All potential risks, side effects, and discomforts.
- Any potential benefits to you or others.
- Alternative treatment options available outside the trial.
- The extent to which your personal health information will be kept confidential.
- Whether any compensation or medical treatments are available if injury occurs.
- Who to contact with questions or if you experience a problem.
- That your participation is completely voluntary and you can withdraw at any time for any reason without penalty or loss of benefits to which you are otherwise entitled.
Should You Join a Clinical Trial? A Guide to Weighing Risks vs. Rewards
Deciding to join a clinical trial is a personal choice that involves your health, time, and hopes. Understanding the potential benefits and risks is essential for making an informed decision.

Potential Benefits of Participation
- Access to New Treatments: Gain access to cutting-edge therapies not yet available to the public, which can be a source of hope when standard treatments have failed.
- Expert Medical Care: Receive close monitoring and care from specialists at top research institutions.
- Active Role in Healthcare: Become a partner in your own care and contribute directly to medical progress.
- Helping Others: Your participation generates data that helps researchers develop better treatments for future patients.
Understanding the Potential Risks
- Unknown Side Effects: Since the treatment is experimental, there may be unexpected side effects, ranging from mild to serious.
- Ineffective Treatment: The new intervention may not work for you, or it may be less effective than existing standard treatments.
- Placebo Group: You could be randomly assigned to a control group, receiving an inactive placebo or standard care instead of the new therapy.
- Time and Effort: Trials often require a significant time commitment for frequent visits, tests, and procedures over months or years.
Critical Questions to Ask Before Joining
Discuss these questions with the research team and your doctor. Never feel pressured to decide quickly.
- What is the purpose of this study?
- What are all of my responsibilities as a participant?
- What are the possible risks and benefits?
- What are my other treatment options?
- How long will the study last, and what is the time commitment?
- Who pays for the treatment and related care?
- How will my privacy be protected?
- What happens if I get sick or have side effects?
- Can I leave the study at any time? (The answer should always be yes.)
The Future Is Now: How Tech Is Revolutionizing Clinical Trials
The world of clinical trials is in the midst of a technological revolution. Innovations are making research more efficient, inclusive, and patient-focused than ever before. At Lifebit, our federated AI platform is at the forefront of this change, enabling secure, real-time analysis of global biomedical data to accelerate drug discovery, optimize trial design, and enhance safety monitoring.
The Critical Importance of Diversity in Trials
Historically, clinical trials have suffered from a severe lack of diversity. For decades, participants were predominantly white men, creating a massive gap in our understanding of how new medicines affect different populations. This is a critical safety issue. People can respond differently to treatments based on their genetics, age, gender, ethnicity, and lifestyle. For example, genetic variations common in certain ethnic groups can affect how quickly a drug is metabolized, potentially leading to a toxic buildup or a lack of efficacy.
Recognizing this, regulatory agencies are pushing for change. The FDA has issued guidance urging sponsors to enroll more participants from underrepresented racial and ethnic populations. The goal is to ensure that trial populations reflect the real-world diversity of the people who will ultimately use the medicine, making treatments safer and more effective for everyone.
The Rise of Decentralized Clinical Trials (DCTs)
Decentralized trials leverage technology to bring the research to the participant, rather than requiring the participant to travel to a central research site. This model dramatically reduces the burden on patients and opens up participation to a much wider and more diverse population.
Key technologies enabling DCTs include:
- Telemedicine: Virtual visits with research staff for consultations and follow-ups.
- Wearable Devices: Smartwatches and other sensors that continuously collect real-world data like heart rate, activity levels, and sleep patterns.
- Electronic Patient-Reported Outcomes (ePROs): Smartphone apps and online diaries that allow participants to report symptoms and quality of life data from home.
- E-consent: Digital platforms for reviewing and signing informed consent documents remotely.
By removing geographic barriers, DCTs make it easier for people in rural areas, those with mobility issues, and individuals with busy schedules to join. This leads to faster recruitment, higher retention rates, and more diverse, generalizable data. However, challenges remain, including the digital divide, ensuring data security and privacy, and integrating data from multiple sources.
Innovations Shaping the Next Generation of Research
Beyond decentralization, several other trends are reshaping the clinical trial landscape:
- Adaptive Trial Designs: Unlike traditional trials with a fixed protocol, adaptive designs allow for pre-planned modifications based on accumulating data. For example, a trial might drop an ineffective treatment arm, adjust the sample size, or focus on a patient subgroup that is responding particularly well. This flexibility can make trials more efficient, ethical, and likely to succeed.
- Precision Medicine and Master Protocols: Instead of a “one-size-fits-all” approach, precision medicine aims to tailor treatments to individuals based on their genetic or molecular profiles. This has led to novel trial designs called master protocols. Basket trials test one drug on multiple diseases that share the same biomarker. Umbrella trials test multiple drugs on one disease that is stratified into subgroups based on different biomarkers. These designs are a more efficient way to test targeted therapies.
- Artificial Intelligence (AI) and Machine Learning: AI is being deployed across the entire trial lifecycle. It can analyze vast datasets to identify novel drug targets, optimize protocol design, predict patient recruitment bottlenecks, identify the best clinical sites, and analyze complex data from imaging and genomics to find subtle patterns.
How to Find a Clinical Trial
Finding the right clinical trial is easier than ever with these resources:
- Your Doctor: They know your medical history and can recommend appropriate studies.
- Patient Advocacy Groups: These organizations often list relevant trials and provide patient-focused support.
- Research Centers and Hospitals: Major medical centers usually have websites with searchable databases of their ongoing trials.
- ClinicalTrials.gov: This comprehensive, official database from the U.S. National Library of Medicine lists trials from around the world. You can search by condition, location, and more.
Your Top 3 Questions About Clinical Trials, Answered
Here are clear answers to the most common questions about participating in a clinical trial.
Can I leave a clinical trial after it starts?
Yes, absolutely. Participation is 100% voluntary. You have the right to withdraw from a study at any time, for any reason, without penalty. Leaving a trial will not affect your standard medical care. This is a fundamental right of every participant.
Who pays for the costs associated with a clinical trial?
Generally, the study sponsor (e.g., a pharmaceutical company) covers costs directly related to the research, including the experimental treatment and special tests required by the protocol. Your health insurance may be billed for routine care you would receive anyway, like standard doctor visits. The informed consent document must clearly explain all financial aspects, so be sure to ask questions if anything is unclear.
What is a placebo?
A placebo is an inactive substance (like a sugar pill) that looks identical to the experimental treatment being tested. It is used as a control to help researchers determine if the new treatment’s effects are real and not just due to patient expectation (the “placebo effect”). The use of placebos is highly regulated to ensure participant safety, and you will always be told if a study involves a placebo group.
Your Role in the Future of Medicine
Every modern medicine is the result of a clinical trial. This complex journey is powered by volunteers who become partners in advancing science for everyone. Their participation has led to life-saving antibiotics, transformative cancer therapies, and protective vaccines.
The future of clinical research is bright, with a focus on inclusivity, accessibility through decentralized trials, and faster processes. Technology is key to this evolution, making research more efficient while upholding strict safety standards.
At Lifebit, we accelerate this progress. Our federated AI platform helps researchers securely analyze global biomedical data in real-time, enabling faster, more informed decisions in clinical trial design and safety monitoring. By using data more effectively, we help bring breakthroughs to patients sooner.
Clinical trials are acts of hope, powered by science and made possible by volunteers. They are how we build a healthier future for all.
Find how federated data platforms accelerate drug findy and safety monitoring


