CMS Data Dive: Finding the Healthcare Information You Need
Why CMS Data is Essential for Healthcare Research and Analysis
CMS data offers researchers and policymakers access to healthcare information for over 160 million Americans covered by Medicare, Medicaid, the Children’s Health Insurance Program (CHIP), and the Health Insurance Marketplace. This repository of enrollment records, claims, provider data, and quality measures is vital for critical healthcare research and policy.
Quick Access Guide to CMS Data:
- Public Data: Free access through Data.CMS.gov – no agreements required
- Research Data: Requires Data Use Agreement (DUA) and fees via ResDAC
- Provider Data: Available through Provider Specific Files and cost reports
- Coverage Information: Search Medicare Coverage Database for policy details
Using this data helps make healthcare more efficient, expands medical knowledge, and supports public health. However, accessing CMS data can be complex for organizations aiming to use it for research, drug development, or population health analysis.
CMS provides two main data types: Public Use Files (PUFs), which are de-identified and free to download, and Research Identifiable Files (RIFs), which contain protected health information and require a formal application. Choosing the right type is key to efficient data access.
I’m Maria Chatzou Dunford, CEO of Lifebit. With over 15 years of experience in computational biology and federated data platforms, I’ve seen how secure access to complex datasets like cms data can transform medical research and patient outcomes, particularly in drug findy and precision medicine.
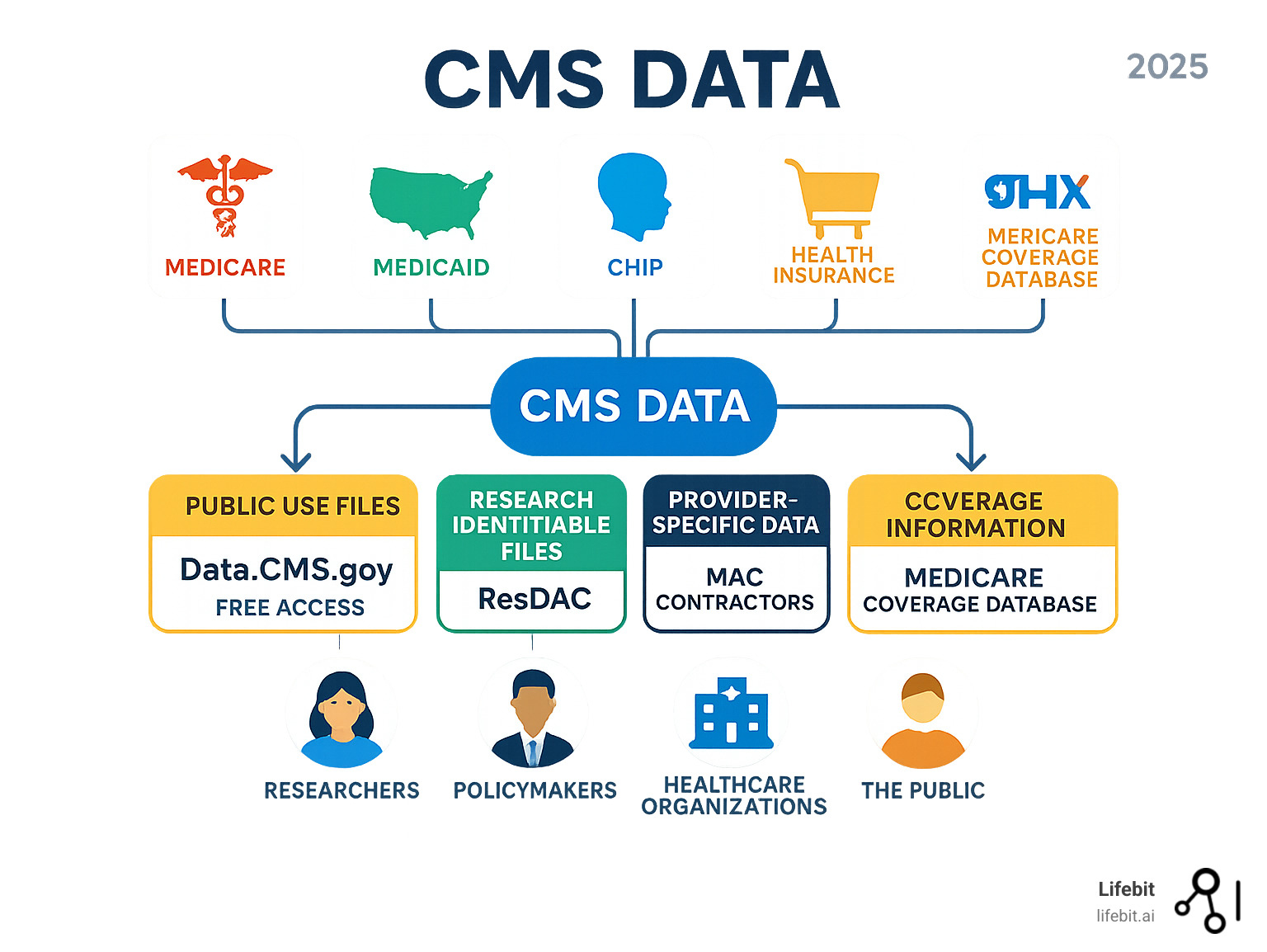
Understanding the Landscape of CMS Data
The Centers for Medicare & Medicaid Services (CMS) manages healthcare data for over 160 million individuals, collecting information from every doctor visit, prescription, and treatment.
CMS data covers several key programs: Medicare (for seniors and disabled individuals), Medicaid & CHIP (for low-income families and children), and the Health Insurance Marketplace (from ACA exchanges). The data’s value lies in its breadth and depth, including:
- Program data (enrollment numbers, service use)
- Enrollment data (coverage details)
- Claims data (specific services, diagnoses, prescriptions)
- Provider data (hospital characteristics, physician specialties)
- Quality measures that track care performance
For those ready to dive in, there are two main portals to explore. Data on CMS Programs offers a comprehensive look at various CMS initiatives, while Data on Medicaid and CHIP provides state-specific insights into these crucial safety net programs.
What Types of Data Files Are Available?
CMS offers three types of data files to suit different research and privacy needs.
Public Use Files (PUFs) are the most accessible. These files are completely de-identified, meaning all personal information is removed. Anyone can download PUFs for free without agreements, making them ideal for preliminary research or trend analysis.
Limited Data Sets (LDS) are also de-identified but retain some indirect identifiers like service dates or partial zip codes. Access requires a Data Use Agreement (DUA) with CMS and provides more detail for sophisticated analysis.
Research Identifiable Files (RIFs) contain identifiable data at the individual level, enabling robust, longitudinal studies. Accessing RIFs is a rigorous process requiring a formal application, a DUA, fees, and often Institutional Review Board (IRB) approval.
| Feature | Public Use Files (PUFs) | Limited Data Sets (LDS) | Research Identifiable Files (RIFs) |
|---|---|---|---|
| Accessibility | Free download, no agreements | Requires DUA | Requires DUA, application, often IRB approval |
| Cost | Free | Typically nominal fees | Fees vary by data size and type |
| Data Content | Aggregated, highly de-identified | De-identified with some indirect identifiers | Individual-level with protected health information |
How CMS Ensures Data Privacy and Security
CMS prioritizes patient privacy through multiple safeguards. The Health Insurance Portability and Accountability Act (HIPAA) provides the legal framework. Data Use Agreements (DUAs) are binding contracts that specify how researchers can use the data. The Data Privacy Safeguard Program (DPSP) enforces strict technical security standards for data environments.
CMS uses sophisticated de-identification methods to protect privacy in PUFs and LDS files. For the most sensitive RIFs, analysis is often restricted to secure access protocols, such as virtual environments, preventing data download. You can learn more about these protections by reviewing the official CMS data privacy policies.
How to Access Publicly Available CMS Data
Publicly available CMS data is the perfect entry point for new researchers or those needing high-level insights. It offers immediate access with no fees or paperwork.
These resources are designed for accessibility, allowing you to download datasets, analyze trends, and use the information for educational purposes without regulatory problems. CMS provides user-friendly portals with Public Use Files (PUFs), which are aggregated and de-identified. You’ll also find interactive dashboards, pre-compiled data tables, and mapping tools to visualize trends.
These public resources work beautifully for initial explorations, understanding population health trends, or getting a feel for the data before diving into more complex research requiring restricted files.
Navigating Data.CMS.gov for Program Information
Data.CMS.gov is the main gateway to public CMS data, including Medicare, special program, and Health Insurance Marketplace information. The site is organized to help you find what you need efficiently. Key datasets on the platform include:
- Medicare Enrollment: Find eligible practitioners and their enrollment records, such as the Ordering and Referring dataset with National Provider Identifiers (NPIs).
- Part D Prescriber Data: Analyze medication trends using tools like the Part D Prescriber Look Up Tool to see which drugs are prescribed to Medicare beneficiaries.
- Market Saturation and Utilization: Use interactive maps to see the density of healthcare providers relative to beneficiaries in different areas.
- Provider Revalidation: Track provider compliance and participation patterns over time.
Ready to start exploring? Visit Explore datasets on Data.CMS.gov to browse these resources.
Searching the Medicare Coverage Database (MCD)
The Medicare Coverage Database (MCD) is the definitive guide to what Medicare covers. It answers whether a specific treatment, device, or service qualifies for reimbursement.
The database includes National Coverage Determinations (NCDs), which are binding nationwide policies, and Local Coverage Determinations (LCDs), which are granular decisions made by regional contractors for their specific jurisdictions.
The MCD allows flexible searching. You can search by keyword (e.g., “telehealth”), document ID, or specific billing codes like CPT/HCPCS for procedures and ICD-10 for diagnoses. You can also filter by state or region to see local coverage variations, which is useful as policies can differ geographically.
Start your search on the MCD to dive into Medicare coverage documents. Whether you’re a healthcare provider verifying coverage details or a researcher studying policy impacts, this database provides the authoritative answers you need.
Accessing Restricted CMS Data for Research
For research that requires more detail than public files offer, restricted CMS data provides granular, patient-level information. Accessing Limited Data Sets (LDS) and Research Identifiable Files (RIFs) enables breakthrough research but requires a more involved process.

The path to accessing restricted data involves several key steps:
- Develop a Research Proposal: Clearly outline your research goals and justify the need for restricted data.
- Obtain IRB Approval: Most projects require approval from an Institutional Review Board (IRB) to ensure ethical standards and protect patient rights.
- Submit a CMS Application: Complete detailed forms and provide all necessary documentation, including IRB approval.
- Sign a Data Use Agreement (DUA): This is a legally binding contract outlining how you will protect and use the data.
- Pay Data Fees: Fees cover the cost of data preparation and maintenance and vary by the request.
The entire process can take several months, but the data’s depth makes it a worthwhile investment for serious research.
The Role of ResDAC in Your Research Journey
The Research Data Assistance Center (ResDAC) is a CMS contractor that helps researchers steer the complex process of accessing restricted CMS data. ResDAC acts as a guide, providing crucial support throughout your research journey. They help you:
- Select the appropriate data files for your research questions.
- Complete the application with templates and instructions.
- Understand the timelines and costs involved.
- Access data documentation, file layouts, and training materials.
- Clarify policies related to DUAs and data privacy.
For anyone considering restricted CMS data, we strongly recommend starting with Learn about requesting RIFs from ResDAC. Their expertise can save significant time and increase your chances of a successful application.
Understanding Key Restricted CMS Data Files
Restricted CMS data includes a rich ecosystem of interconnected files. Understanding the key files is essential for maximizing your research.
- Master Beneficiary Summary File (MBSF): The core file for Medicare research, containing beneficiary demographics, enrollment history, and health status. It’s the key to linking other Medicare files.
- Medicare Claims Data: Includes detailed files for inpatient (MedPAR), outpatient, physician/supplier (Part B), home health, skilled nursing facility, and hospice services.
- Medicare Part D Data: Provides comprehensive prescription drug information, including medications, costs, and prescribers, essential for drug utilization studies.
- Medicaid T-MSIS Data: Offers comprehensive data on Medicaid and CHIP beneficiaries, claims, and managed care encounters from the Transformed Medicaid Statistical Information System.
- Assessment and Survey Data: Clinical data from instruments like the Minimum Data Set (nursing homes) and self-reported patient information from the Medicare Current Beneficiary Survey (MCBS) add crucial context.
The real power comes from linking these files to create a complete picture of a patient’s care journey across different settings and programs.
Key Provider Datasets and How to Use Them
Provider data is as crucial as patient data for a complete healthcare picture. It reveals who delivers care, where they are located, and how they are paid.
This data allows researchers to analyze provider characteristics, service provision, payment structures, and regulatory compliance, connecting patient outcomes to the care delivery environment.
Finding and Using Provider Specific Files (PSF)
The Provider Specific File (PSF) contains the unique provider characteristics Medicare uses to calculate payments under the Prospective Payment System (PPS). Maintained by regional Medicare Administrative Contractors (MACs), the PSF covers all facility types, from hospitals to hospices.
The files are available in user-friendly CSV format, and you can access historical PSF data to begin your analysis.
Leveraging Other Essential Provider CMS Data
While PSF data is valuable, several other cms data sources offer different angles on the healthcare delivery system.
The Healthcare Cost Reporting Information System (HCRIS) contains annual cost reports from Medicare-certified providers. It includes facility characteristics, utilization data, costs, and financial statements, making it essential for studying healthcare economics. You can find hospital cost reports in this system.
The National Plan and Provider Enumeration System (NPPES) assigns a unique National Provider Identifier (NPI) to each provider. The associated taxonomy codes identify a provider’s specialty, which is invaluable for linking different datasets.
The Provider of Services (POS) File contains demographic and service details for non-laboratory providers, helping researchers identify characteristics like ownership and facility size.
Linking these provider datasets creates a comprehensive view essential for studying provider behavior, facility impact on outcomes, and the relationship between healthcare structure and performance.
Frequently Asked Questions about CMS Data
Navigating cms data can be complex. Here are answers to some of the most common questions researchers have.
What are the main benefits of using CMS data for research?
The primary benefits of using cms data stem from its scale and authenticity:
- Large Population Coverage: With data from over 160 million people, you can study rare conditions and achieve statistical power.
- Longitudinal Tracking: The data follows individuals for years, allowing for long-term studies on disease progression and treatment outcomes.
- Real-World Evidence: It reflects healthcare as it’s actually delivered, providing an authentic picture of treatment effectiveness outside of controlled trials.
- Policy Evaluation: It enables robust analysis of how policy changes affect patient access, costs, and outcomes.
This combination makes cms data essential for research that can improve patient care.
What is the difference between Public Use Files (PUFs) and Research Identifiable Files (RIFs)?
These are two distinct levels of data access designed for different needs:
- Public Use Files (PUFs): These are de-identified or aggregated files that protect patient privacy. They are free, downloadable from Data.CMS.gov without special agreements, and ideal for preliminary analysis or studying broad trends.
- Research Identifiable Files (RIFs): These files contain protected health information (PHI) at the individual patient level, enabling detailed longitudinal studies. Access requires a formal application, a Data Use Agreement (DUA), fees, and often IRB approval.
The right choice depends on your research question. Many researchers start with PUFs to refine their approach before applying for RIFs.
How often is CMS data updated?
Update schedules for cms data vary by dataset.
- Public Use Files (PUFs) are typically updated annually, with data becoming available about 18 months after the calendar year ends.
- Research Identifiable Files (RIFs) are often updated more frequently, with some available quarterly and others annually.
- Real-time data is limited, though some systems allow for real-time eligibility verification.
Always check the documentation for the specific dataset you are interested in to understand its update cycle. This is crucial for planning your research timeline, especially when factoring in the application process for restricted data access.
Conclusion
Leveraging CMS data enables transformative research that can improve patient lives. This guide has covered the landscape of available information, from publicly accessible summary statistics to the detailed patient records in restricted files that power groundbreaking findies.
The journey begins with matching your research needs to the right data type: Public Use Files (PUFs) for initial exploration, Limited Data Sets (LDS) for more granular insights, and Research Identifiable Files (RIFs) for deep, individual-level analysis. We’ve highlighted key resources like Data.CMS.gov for public data, the Medicare Coverage Database for coverage policies, and ResDAC as an essential guide for accessing restricted files.
CMS’s commitment to patient privacy, through safeguards like HIPAA and DUAs, enables ethical research by building trust. The power of CMS data lies in its scope and authenticity. With over 160 million individuals represented, longitudinal tracking capabilities, and real-world evidence from actual clinical practice, this data drives meaningful health outcomes research and policy evaluation that shapes healthcare for everyone.
Analyzing sensitive datasets like cms data at scale requires a sophisticated data infrastructure. Organizations need platforms that manage security, enable advanced analytics, and facilitate collaboration under strict governance. Mastering access to CMS data positions you to contribute to research that improves healthcare outcomes. The future of medicine depends on our ability to securely extract insights from complex data, and CMS data is one of our most valuable resources in this mission.

