Remote Control: How to Recruit Patients for Decentralized Trials

Remote Trial Recruitment: 5 Moves to Enroll Faster, Cut Costs, and Reach Patients You’re Missing
Remote clinical trial recruitment is the process of identifying, engaging, and enrolling patients into clinical studies using digital tools and decentralized methodswithout requiring them to travel to a physical research site. Here’s what you need to know:
Key Components of Remote Clinical Trial Recruitment:
- Digital Outreach – Using social media, patient registries, and online communities to find eligible participants
- Virtual Screening – Pre-qualifying patients through online questionnaires and telehealth assessments
- eConsent – Obtaining informed consent remotely via video calls and electronic signatures
- At-Home Participation – Enabling patients to complete study activities from their homes using wearables, mobile apps, and mail-in kits
- Continuous Engagement – Maintaining participant involvement through digital platforms and remote monitoring
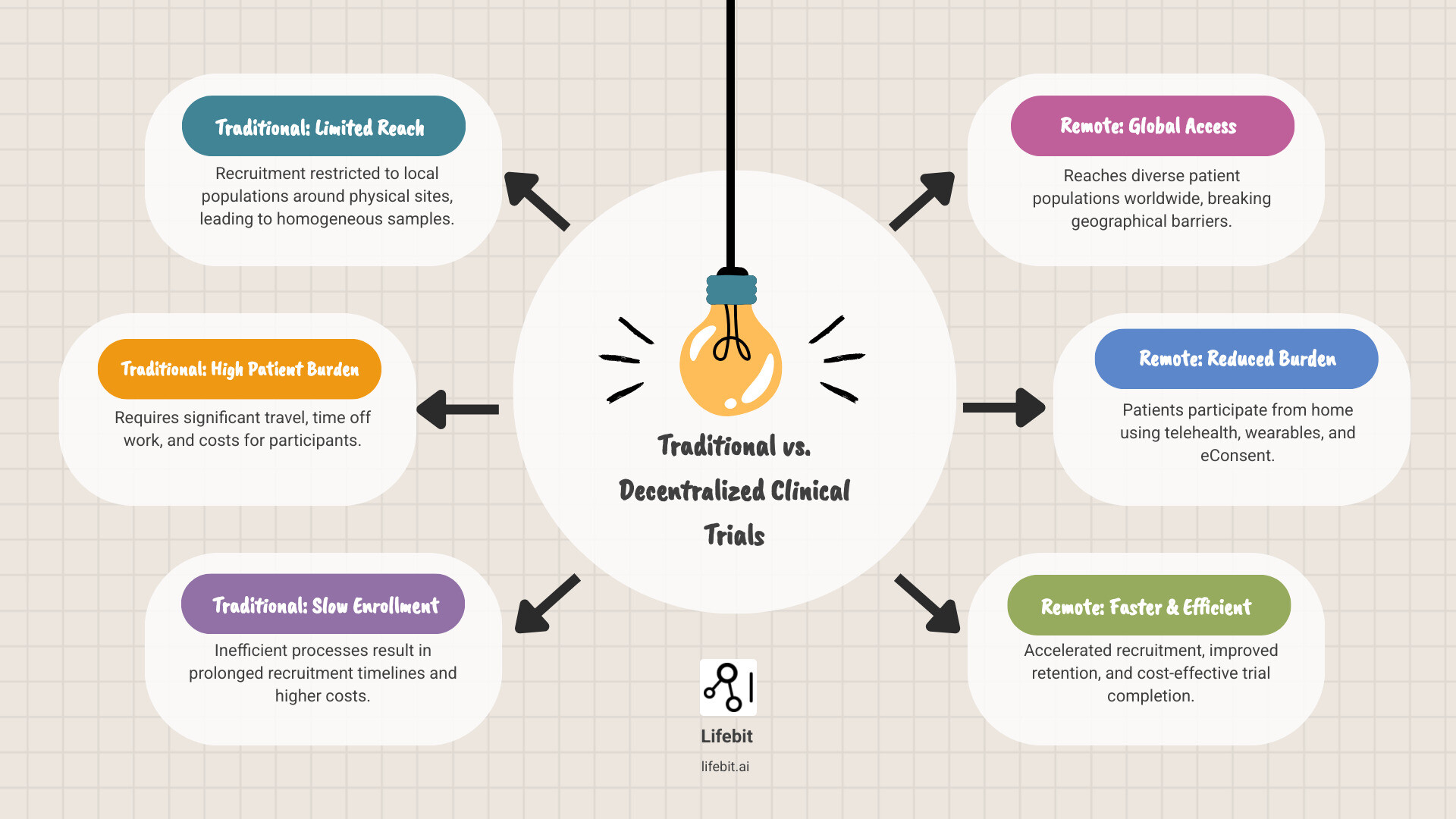
Traditional clinical trials face a harsh reality: nearly all are conducted at physical sites, limiting recruitment to people who live nearby. This approach struggles to enroll large, diverse, representative samples. Multi-site trials can help, but they require massive infrastructure and budgets that many sponsors can’t afford.
The result? Slow enrollment, high costs, homogeneous participant pools, and trials that fail to reflect the real-world populations who will ultimately use these treatments. For rare diseases or underserved communities, participation becomes nearly impossible.
Decentralized Clinical Trials (DCTs) change everything. By leveraging telehealth, mobile health technologies, and remote patient monitoring, DCTs remove geographical barriers and bring the trial to the patientnot the other way around. This shift isn’t just convenient; it’s essential for creating more inclusive, efficient, and scientifically valid research.
As CEO and Co-founder of Lifebit, I’ve seen how our federated data platforms power secure, compliant remote clinical trial recruitment. My background in computational biology and AI has shown me that the right technology can open up access to diverse patient populations while upholding the highest standards for data quality and regulatory compliance.
Remote clinical trial recruitment definitions:
- AI clinical trial recruitment
- clinical trial patient recruitment companies
- clinical trial patient support
Site-Based Recruiting Is Costing You Months and Diversity—Remote Trials Fix It Now
Imagine a patient with a chronic condition, living hours from a research center. A promising clinical trial appears, but it requires long drives, time off work, and childcare for multiple visits. For most, this is impossible.
This scenario plays out thousands of times across traditional clinical trials. The geographical limitations alone eliminate most potential participants before they even consider enrolling. Nearly all clinical trials are conducted at physical sites, which means recruitment is limited to people who live nearby and have the resources to make repeated visits. For someone in a rural area, or someone juggling multiple jobs, or someone without reliable transportation, participation becomes impossibleno matter how promising the treatment might be.
The financial burden is also immense. Travel, parking, and lost income can total thousands of dollars, creating a significant barrier for many families.
The result? Slow recruitment timelines that can delay trials by months or even years. High dropout rates as participants struggle to maintain the demanding schedule. And perhaps most troubling, a fundamental lack of sample representativeness. When only patients near major academic medical centers can participate, the study population doesn’t reflect the broader patient community. How can we trust that a treatment will work for everyone when it’s only been tested on a narrow slice of the population?
Multi-site trials help, but they bring soaring infrastructure costs and complex coordination, while still facing geographical barriers.
Remote clinical trial recruitment flips this entire model on its head. Instead of asking patients to come to the trial, we bring the trial to the patients. Telehealth visits replace in-person appointments. Wearable devices collect data continuously without requiring a single trip to a clinic. Home-based sample collection kits arrive by mail. Electronic consent forms can be reviewed and signed from the comfort of a patient’s couch.
This isn’t just about convenience (though that matters enormously). It’s about fundamentally expanding who can participate in medical research. A working parent in a small town can now join a trial without disrupting their entire life. Someone with mobility challenges doesn’t need to steer transportation logistics. Patients with rare diseaseswho might be the only person with their condition within a hundred-mile radiuscan suddenly connect with researchers anywhere in the world.
The impact on diversity and representativeness is profound. We’re no longer limited to recruiting patients who happen to live near research institutions. We can reach across geographical, socioeconomic, and demographic boundaries to build study populations that actually reflect the real world. The value of decentralized clinical trials extends far beyond logisticsit fundamentally improves the quality and applicability of research.
Breaking Down Geographical and Demographic Barriers
Rural patients have always been underrepresented in clinical research, not because they’re unwilling to participate, but because participation has been practically impossible. Remote clinical trial recruitment changes this equation entirely. A farmer in Montana can participate just as easily as someone living next door to a major hospital. This matters especially for rare disease populations, where potential participants might be scattered across entire continents.
The ripple effects are significant. When we remove geographical barriers, we naturally increase diversity in our study samples. We reach patients across different ages, ethnicities, income levels, and cultural backgrounds. We include people from underserved communities who’ve historically been left out of medical research. This isn’t just the right thing to do from an equity standpointit’s scientifically essential.
Better sample diversity leads to improved study generalizability. When trial results come from a truly representative population, we can be more confident that treatments will work safely and effectively for all patients, not just a narrow subset. This is health equity in action: ensuring that medical advances benefit everyone, not just those with the resources and proximity to participate in traditional trials.
The Benefits for Participants, Sponsors, and CROs
The beauty of remote trials is that everyone wins. Let’s break down what this looks like for each group:
Participants experience unprecedented convenience. They can fit trial activities around their lives instead of rearranging everything to accommodate site visits. Morning telehealth check-ins can happen before work. Wearable devices collect data while they sleep. Symptom questionnaires can be completed during lunch breaks. This dramatic reduction in burden translates directly into lower dropout rateswhen participation fits naturally into daily life, people are far more likely to see the trial through to completion.
Sponsors gain speed and efficiency. Recruitment happens faster when you’re not limited by geography. Trials complete more quickly when dropout rates plummet. And while there’s an upfront investment in digital infrastructure, the long-term cost savings can be substantial. Fewer site visits mean lower site management costs. Digital data collection reduces manual data entry and associated errors. Faster completion means treatments reach patients sooner.
CROs achieve greater operational efficiency by streamlining workflows through digital tools. Patient management becomes more systematic. Data collection happens in real-time rather than waiting for site visits. Monitoring can be centralized rather than requiring staff to travel to multiple sites. This allows CROs to manage larger, more complex studies while maintainingor even improvingdata quality.
The traditional model wasn’t broken because anyone did something wrong. It was broken because it was designed for a different era, before we had the technology to do better. Now we do. And the results speak for themselves: faster enrollment, better retention, more diverse samples, and ultimately, better science.
Remote Trial Recruitment Playbook: 3 Steps to Fill Your Study Fast
So how do you actually build a successful remote clinical trial recruitment strategy from the ground up? It’s not as simple as posting a Facebook ad and waiting for participants to roll in. The most effective approach requires a thoughtful digital strategy that guides potential participants from initial awareness all the way through patient identification, pre-screening, and final enrollment.
Think of it as a patient-centric funnel. Every touchpoint, from the first ad to the final eConsent, matters. A well-designed process not only accelerates enrollment but also attracts more diverse and committed participants.

Step 1: Finding and Engaging Patients Online
The internet has fundamentally changed how we find people. In the past, recruitment meant flyers in hospital waiting rooms and word-of-mouth referrals. Today, we can reach thousands of potential participants with the right message at exactly the right moment in their healthcare journey.
Digital marketing forms the foundation of modern recruitment. Targeted online advertising campaigns let us reach specific patient populations based on demographics, interests, and even health-related online behavior. But the real magic happens when we combine multiple channels strategically.
Social media advertising on platforms like Facebook, Instagram, and Twitter offers unprecedented targeting capabilities. You can reach people who’ve shown interest in specific health conditions, joined relevant groups, or engaged with similar content. That said, crafting these messages requires care and sensitivityespecially for conditions like mental health disorders where stigma and misunderstanding are already significant barriers. The goal is always to inform and invite, never to manipulate or pressure.
Beyond paid advertising, patient advocacy groups represent some of the most valuable partnerships in remote clinical trial recruitment. These organizations have spent years building trust within their communities. When they share information about a trial, it carries weight. Their newsletters, forums, and events provide authentic channels to reach patients who are actively engaged in managing their conditions.
Then there are online health communities like HealthUnlocked, the world’s largest social network for health, which hosts communities for over 300 different conditions. These platforms are where patients go to share experiences, ask questions, and find support. Engaging respectfully and transparently in these spaces can connect you directly with motivated individuals who are already seeking ways to contribute to research.
Finally, don’t overlook dedicated patient registries and research matching platforms. ResearchMatch and similar services exist specifically to connect willing participants with relevant studies. These individuals have already expressed interest in contributing to research, making them some of the most motivated potential participants you’ll find.
One crucial tip: when crafting your outreach messages, avoid listing specific eligibility criteria in your ads. This might seem counterintuitive, but stating requirements upfront can lead to self-selection bias. Instead, focus on the broader opportunity to contribute to science and encourage interested individuals to complete an initial screening questionnaire.
Step 2: Using AI for Smarter Pre-Screening and Matching
Once you’ve attracted potential participants, the next challenge is figuring out who actually qualifiesand doing it efficiently. This is where artificial intelligence transforms remote clinical trial recruitment from a manual slog into a streamlined, data-driven process.
Traditional pre-screening is a manual, time-consuming, and error-prone review of lengthy questionnaires against complex criteria.
AI-powered matching changes everything. These systems can analyze responses from automated pre-screening questionnaires in real-time, applying sophisticated logic to determine likely eligibility. They can even integrate omnichannel datapulling in information from patient registries, electronic health records, and digital marketing interactionsto build a more complete picture of each potential participant.
The result? You identify qualified candidates faster, with greater accuracy, and without burning out your recruitment team. More importantly, this data-driven approach to patient identification helps ensure enrollment quality. You’re not just filling slots; you’re finding the right people for each specific study, increasing the likelihood they’ll remain engaged throughout the trial.
Step 3: Mastering Remote Informed Consent (eConsent)
Informed consent is the ethical foundation of clinical research. A key question for remote trials was whether meaningful consent could be obtained without face-to-face interaction.
The answer, backed by research and real-world experience, is a resounding yes. In fact, remote informed consent through eConsent platforms often leads to better outcomes than traditional paper-based approaches.
The eConsent process typically unfolds in several steps. First, participants receive digital consent materials through a secure online platform. Unlike static paper forms, these digital versions can include videos, interactive diagrams, and other multimedia elements that improve comprehension. Complex concepts like randomization or potential side effects become much clearer when you can show them visually.
But here’s what really makes eConsent work: the human connection isn’t lost. A critical component is a video conferencing session with a member of the research team. This real-time conversation allows participants to ask questions, seek clarification, and discuss any concernsjust like they would in person. Some platforms even offer shared screen control, so the research coordinator can walk participants through each section of the consent form together.
Once all questions are answered and the participant feels fully informed, they provide consent via secure electronic signatures. These digital signatures are legally binding and must comply with regulations like 21 CFR Part 11 for FDA-regulated trials, as well as data privacy laws like HIPAA and GDPR.
Research on remote consenting modalities has shown that electronic approaches actually improve patient comprehension, usability, and workflow compared to standard paper-based methods. Participants can take their time reviewing materials, revisit sections as needed, and feel more empowered in the decision-making process.
The technology behind eConsent must be rock-solid, of course. Security, compliance, and data integrity are non-negotiable. But when implemented properly, remote informed consent doesn’t just match the quality of in-person consentit often exceeds it.
Remote Trial Tech Stack: Tools to Scale Enrollment and Capture Real‑Time Data
A successful remote clinical trial recruitment campaign relies on a sophisticated ecosystem of digital tools. This tech stack acts as the nervous system of a decentralized trial, connecting patients, researchers, and data.
The right tech stack improves the entire research experience. Participants can use smartphones, data flows securely from wearables, and virtual visits feel personal. This isn’t just about remote replication; it’s about fundamental improvement.

Essential Platforms for your remote clinical trial recruitment strategy
The foundation of any remote trial begins with platforms that enable secure, compliant digital interactions. eConsent platforms sit at the heart of this ecosystem, allowing participants to review study information, ask questions via video, and provide legally binding electronic signatures from anywhere. REDCap, for instance, has become a global standard, with over 4,600 institutions across 139 countries using it for research functions including eConsent. For FDA-regulated studies, these platforms must comply with 21 CFR Part 11 for electronic signatures. The FDA has even developed its own toolyou can explore FDA guidance on the COVID MyStudies App to see how regulatory bodies are embracing digital-first approaches.
Once participants are enrolled, Electronic Clinical Outcome Assessments (eCOA) take over the day-to-day data collection. These digital tools replace paper diaries and questionnaires, allowing participants to report symptoms, side effects, and quality of life measures directly through web forms or mobile apps. The result? More accurate data captured in real time, without the recall bias that plagues traditional methods.
Telehealth services have transformed how researchers interact with participants. Video conferencing platforms enable face-to-face assessments without the face-to-face travel. Clinicians can conduct study visits, review medication compliance, and build rapport with participantsall through secure video connections. These aren’t just convenient alternatives; they’re often preferred by participants who appreciate the flexibility.
Data capture systems like REDCap, Qualtrics, and SurveyMonkey are invaluable for building surveys. Integrating them with communication tools like Twilio for automated reminders keeps participants engaged and makes remote trials practical at scale.
Leveraging Wearables and Remote Monitoring
Here’s where remote trials get really exciting: the ability to capture continuous, real-world data that would be impossible in traditional settings. Wearable devices have moved beyond fitness tracking to become legitimate research tools, capable of measuring everything from heart rate variability to sleep architecture to daily activity patterns.
These devices generate digital biomarkersobjective, quantifiable physiological data collected passively as participants go about their daily lives. Instead of a single blood pressure reading taken in a clinic (when participants might be anxious or stressed), researchers can now see patterns across days, weeks, or months. The field of remote patient monitoring tools is expanding rapidly, and many devices now capture biomarkers with clinical-grade accuracy.
The power of continuous data collection is immense. While traditional trials offer snapshots, wearables provide a continuous movie of a participant’s health. This data density reveals insights that intermittent clinic visits miss, showing how treatments work in real life.
Even biological samples have gone remote. At-home sample collection kits now enable participants to collect saliva, blood (using innovative tools like the Mitra Blood Collection Cartridge), or urine samples at home and mail them to central labs. These kits come with clear instructions, pre-labeled packaging, and prepaid shipping, making the process surprisingly straightforward.
This combination of technologiesfrom wearables to mail-in kitsdramatically increases both the number and frequency of data points researchers can collect. More importantly, it does so while reducing the logistical burden on participants, who no longer need to schedule their lives around clinic visits. The result is richer data, happier participants, and trials that better reflect how treatments perform in the real world.
Stop Data Risk Before It Starts: How Remote Trials Stay Secure and Fraud‑Resistant
The promise of remote clinical trial recruitment comes with great responsibility. Moving trials into patients’ homes requires a new approach to data quality, fraud prevention, privacy, and security. These are fundamental to research integrity and patient safety.
The good news is that decentralizing technologies also provide powerful tools to manage these risks. Remote trials can offer superior oversight through real-time monitoring and automated safeguards.
Mitigating Data Quality and Fraud Risks in remote clinical trial recruitment
Fraud and data quality issues also exist in traditional trials. Online, these risks evolve. The challenge is to distinguish genuine participants from bots, duplicates, or individuals misrepresenting themselves.
Digital identity verification forms our first line of defense. We collect IP addresses, deploy CAPTCHA tests to weed out automated bots, and verify phone numbers and physical addresses. These measures help confirm that the person on the other end is real and unique.
But verification alone isn’t enough. We also need to look at how people respond. Automated data validation built into our screeners and surveys can flag suspicious patternslike someone completing a 20-minute questionnaire in two minutes, or “straight-lining” through every question with identical answers. We can embed hidden survey items that only genuine participants would answer logically, creating additional tripwires for fraudulent entries.
Centralized monitoring takes this further. Instead of waiting for problems to surface, we continuously scan recruitment data for unusual patterns or outliers. If we suddenly see a spike in enrollments from a particular region, or notice multiple applicants sharing similar demographic profiles with suspiciously similar responses, our systems flag them for review.
Finally, secure audit trails document every interaction and data modification. This transparency creates accountability and allows us to trace any questionable data back to its source. Think of it as a digital paper trail that’s actually better than papersearchable, tamper-proof, and always available.
By layering these technical safeguards with thoughtful protocol design and ongoing human oversight, we create a robust defense against both fraud and honest mistakes that could compromise data quality.
Ensuring Security and Compliance in a Decentralized World
Patient privacy and data security aren’t negotiablethey’re the foundation of trust in clinical research. In a decentralized environment where data flows across multiple platforms, devices, and geographic locations, protecting that data becomes more complex but also more critical.
Remote trials generate vast data from many sources: wearables, apps, video calls, and home-based kits. Each touchpoint is a potential vulnerability that requires active security measures.
HIPAA compliance (in the U.S.) and GDPR considerations (in Europe and beyond) set the regulatory floor, but we aim higher. Every platform we use must implement end-to-end encryption, ensuring that data is protected not just at rest but throughout its entire journey. When a participant submits information through a mobile app, it’s encrypted on their device, remains encrypted during transmission, and stays encrypted in our secure data storage systems.
Secure data storage means more than just a locked server room. We use cloud infrastructure with multiple layers of protectionfirewalls, intrusion detection systems, regular security audits, and access controls that limit who can view sensitive data to only those with a legitimate need to know.
This is where federated data governance becomes especially powerful. Rather than moving all patient data to a central location (which creates a single point of vulnerability), federated approaches allow data to remain distributed across secure environments. Analytics and AI models can be deployed to where the data lives, generating insights without exposing the raw, identifiable patient information. It’s a fundamental shift in how we think about data securityinstead of bringing data to the analysis, we bring the analysis to the data.
| Risk/Challenge | Traditional Trials (Site-based) | Remote Trials (Decentralized) | Mitigation Strategy |
|---|---|---|---|
| Data Quality | Controlled environment, trained staff conduct assessments | Variable home environments, self-reported data | Automated validation, continuous monitoring, clear participant training |
| Identity Verification | In-person verification at site | Online verification required | Digital identity checks, IP tracking, multi-factor authentication |
| Fraud Detection | Manual screening by site staff | Potential for duplicate enrollments, bots | AI-powered anomaly detection, hidden validation items, secure audit trails |
| Data Security | Physical site security, paper records | Data transmitted across networks and devices | End-to-end encryption, secure platforms, access controls |
| Privacy Compliance | Site-level HIPAA procedures | Multi-jurisdictional requirements (HIPAA, GDPR) | Federated governance, encrypted storage, regular compliance audits |
| Patient Safety Monitoring | Site-based clinical examinations, regular visits | Remote monitoring, virtual check-ins | Real-time data monitoring, telehealth consultations, automated safety alerts |
Platforms like Lifebit’s federated AI solution are built specifically to address these challenges. By enabling secure, compliant analysis across distributed datasets, we can harness the power of remote clinical trial recruitment and decentralized data collection while maintaining the highest standards of patient privacy and data security. More info about secure data platforms.
The shift to remote trials doesn’t mean compromising on safety or qualityit means reimagining how we protect both in a digital-first world.

