AI and RWE A Powerful Partnership for Healthcare Innovation
Introduction: The $300 Million-Dollar Question in Pharma
The healthcare world is changing fast, powered by the partnership between ai for real-world evidence (RWE). This combination is open uping insights that were once unimaginable.
Here’s what you need to know:
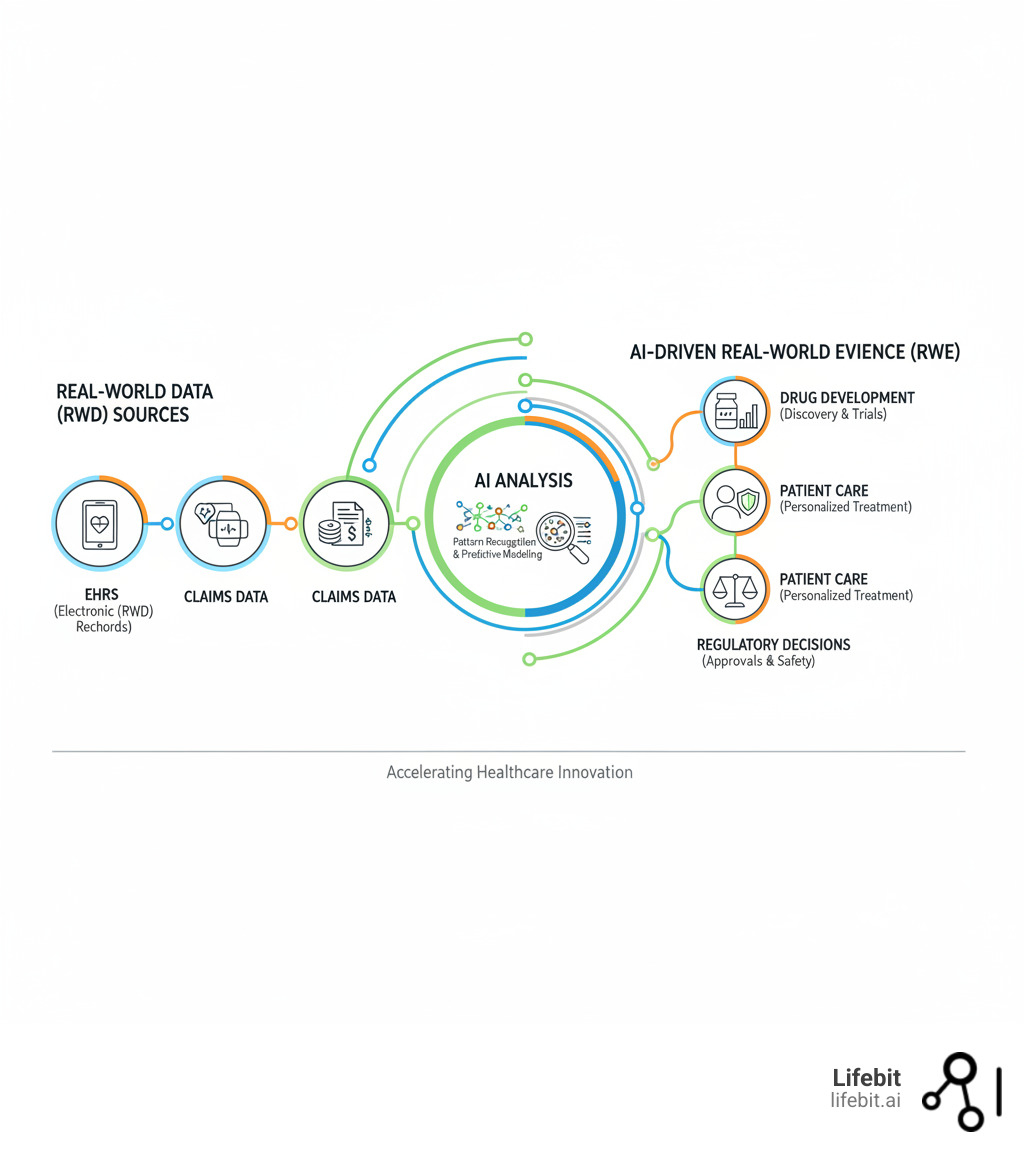
- Real-World Evidence (RWE) comes from data gathered outside clinical trials—like electronic health records (EHRs), insurance claims, and wearables. It shows how treatments work in the real world.
- Why RWE Matters: It provides a deeper understanding of treatments and patient outcomes than trials alone, driving better clinical decisions, regulatory approvals, and drug safety.
- AI’s Role: AI makes sense of this massive, complex data. It finds hidden patterns and predicts outcomes, turning raw data into valuable RWE.
Imagine every piece of patient information contributing to a clearer picture of health. That’s the promise of AI-driven RWE. But the volume of data is overwhelming for traditional analysis. AI automates the grunt work, letting experts focus on findy. The impact is huge: McKinsey projects a top-20 pharma company could generate an extra $300 million annually by integrating advanced RWE analytics.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With 15 years in computational biology and AI, my work has focused on leveraging ai for real-world evidence in secure environments to accelerate biomedical findy and improve patient care.
The AI Revolution: How Machines Are Making Sense of Healthcare’s Messiest Data
Healthcare data is a mess—scattered, unstructured, and locked in silos. For years, valuable insights have been trapped in data that nobody could effectively analyze.
This is where ai for real-world evidence becomes transformative. It’s not just about speed; it’s about changing what’s possible.
Traditional RWE analysis was limited to structured data, leaving the rich narratives in clinical notes invisible. Manual extraction was slow, expensive, and couldn’t scale. At Lifebit, we see how a modern data lakehouse architecture, combining automated data extraction with end-to-end data management, open ups the full spectrum of real-world data. This approach improves privacy by minimizing human intervention, enables interoperability, and accelerates the path from data to clinical validation. The result? Researchers can focus on innovation, not data cleaning.
The Power of Language: Using NLP and LLMs to Open up Unstructured Data
Most critical patient information lives in unstructured text: clinical notes, pathology reports, and patient-reported outcomes. These narratives are gold mines of insight that were previously impossible to analyze at scale.
Natural Language Processing (NLP) and Large Language Models (LLMs) are changing this. They act as translators, reading thousands of medical records to extract and structure meaningful information with clinical accuracy. NLP is especially valuable for phenotyping patients—identifying specific patient groups from text.
LLMs take this even further. As highlighted in recent advances in clinical oncology, they are replacing older NLP systems with better accuracy. At Lifebit, we see LLMs offering “universal structuring” for health information—acting as universal labelers, translators between data standards, and even providing universal reasoning. Explainable AI is crucial; AI that can’t explain its conclusions won’t be trusted or used.
From Data to Decisions: Deep Learning for Predictive Modeling
Extracting data is half the battle; the real magic is using it to predict what’s next.
Deep Learning (DL) excels at this predictive modeling. By analyzing millions of patient records, DL models can predict treatment outcomes, identify patients at risk for toxicity, and enable personalized medicine. A key development is multimodal data integration, where AI weaves together images, genomics, pathology slides, and clinical notes for a complete patient picture.
The results are powerful. In ovarian cancer, combining data types improves risk stratification. For lung cancer, AI analysis of CT scans can predict EGFR mutations with 75-81% accuracy without a biopsy. This represents a fundamental shift from understanding what happened to predicting what will happen.
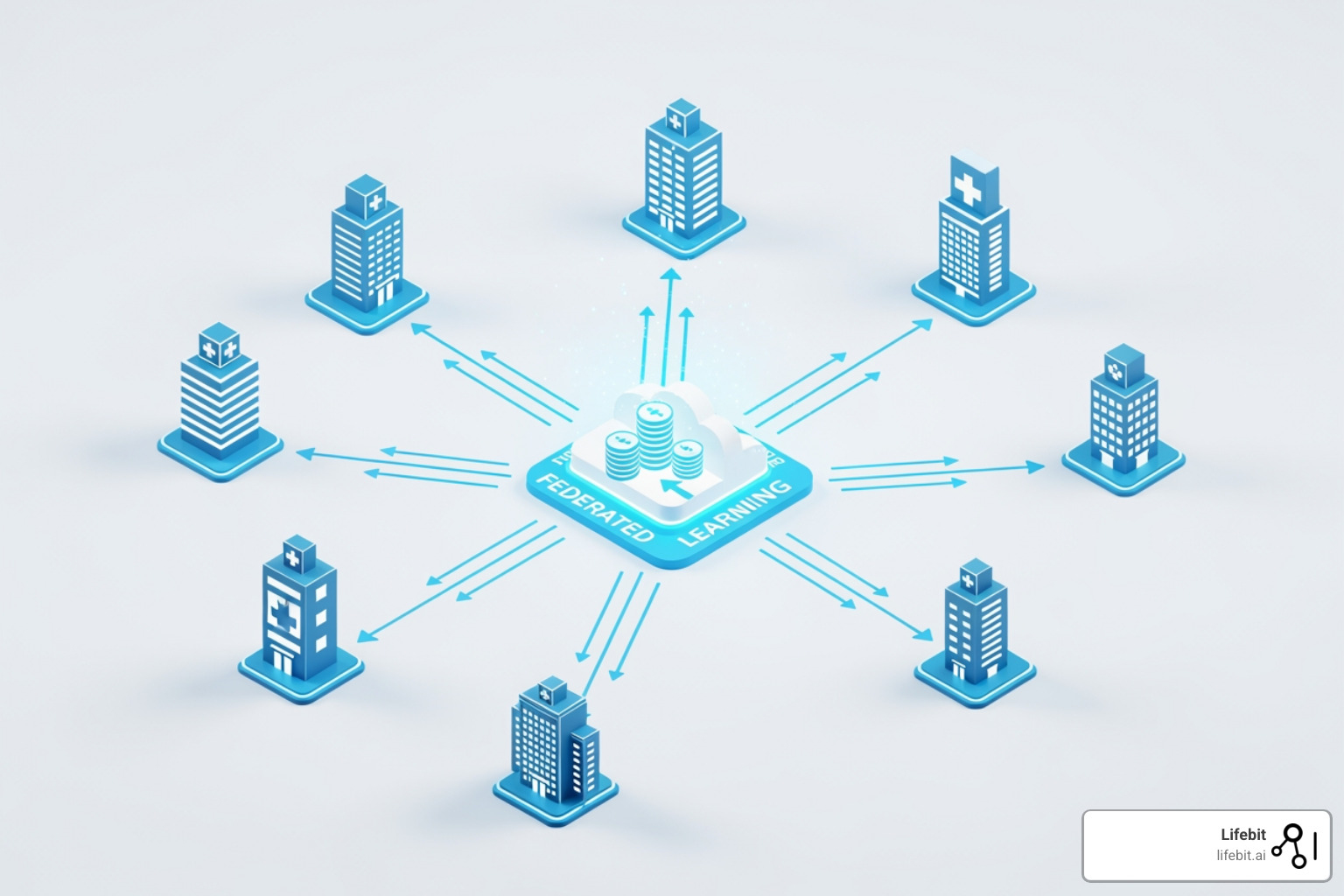
Breaking Down Barriers: Overcoming Data Silos with Federated Learning
The paradox of healthcare research is needing vast, diverse datasets that are locked away in separate institutions by privacy laws like GDPR and HIPAA. You can’t just pool all the data in one place.
Federated learning solves this. Instead of bringing data to the model, it brings the model to the data. The model trains locally at each institution, learning from the data without it ever leaving. Only the aggregated insights are shared.
This approach preserves data security while enabling collaboration across institutions in the UK, USA, Canada, Europe, and beyond. Federated governance ensures each data custodian maintains control. At Lifebit, our federated AI platform is built for this challenge, providing secure access to global biomedical data. This enables large-scale research that produces reliable ai for real-world evidence while respecting patient privacy and regulatory compliance.
From Lab to Life: The Impact of AI for Real-World Evidence
The integration of ai for real-world evidence is a paradigm shift impacting every stakeholder in healthcare: biopharma, payors, providers, policymakers, and patients.

For Biopharmaceutical Companies: Slashing Timelines and Boosting Success
Bringing a new drug to market is slow, expensive, and uncertain. AI for real-world evidence is a game-changer. McKinsey’s research on creating value from next-generation real-world evidence shows a top-20 pharma company can generate an additional $300 million annually with advanced RWE analytics.
AI-driven RWE optimizes drug findy and trial design by identifying responsive patient populations. Post-market safety surveillance becomes faster and more comprehensive. For market access, robust RWE on a drug’s real-world effectiveness is critical for reimbursement negotiations. By automating tedious data tasks, AI frees scientists to focus on innovation, generate targeted evidence, and ultimately improve patient access to new treatments.
For Providers and Payors: Driving Value-Based Care
Providers and payors must balance quality care with cost management. AI for real-world evidence enables true value-based care.
Payors can use AI-driven insights to make confident reimbursement decisions based on how treatments perform in their actual patient populations. This leads to smarter spending on interventions that deliver real value.
For providers, AI-powered clinical decision support is changing daily practice. It can predict which patients are at high risk for adverse events, allowing for proactive intervention. In pharmacovigilance, AI models that detect adverse drug reactions from EHR data are revolutionizing drug safety. While AI for treatment planning is still evolving, it can already identify high-risk patients to prompt crucial conversations about goals of care, and it allows for more precise population health management.
For Policymakers and Patients: Shaping a Healthier Future
The impact of ai for real-world evidence extends to policymakers and patients.
Policymakers gain the insights needed for informed resource allocation and health policy. Public health monitoring becomes faster and more granular. Regulatory decisions become more evidence-based, ensuring approved treatments work for diverse, real-world populations.
Crucially, AI can help address health equity. AI-powered analytics for various stakeholders are being used to assess health disparities. Causal AI can quantify how social determinants of health impact outcomes, allowing for more targeted and equitable interventions.
For patients, this means empowerment. When treatment decisions are informed by RWE from people like them, they can make better healthcare choices. The system becomes more transparent, responsive, and focused on what matters: helping people live healthier lives.
Oncology’s New Weapon: Using AI and RWE to Fight Cancer
The daily practice of oncology is a battle against uncertainty. With over 20 million new cancer cases each year, the need for more precise tools is urgent. AI for real-world evidence is emerging as a critical new weapon for cancer research and precision oncology.
AI-Powered Cancer Screening, Diagnosis, and Staging
Early and accurate detection is paramount. AI is rapidly changing these areas through advanced medical imaging and digital pathology. Convolutional Neural Networks (CNNs) have shown impressive accuracy in skin cancer detection, sometimes outperforming human experts, with predictive values in the 0.80–0.95 range. In colorectal cancer screening, CNNs have been shown to cut the endoscopic miss rate of adenomas in half.
Beyond diagnosis, AI is revolutionizing radiographic image interpretation. A 3D CNN called Sybil accurately detects short-term lung cancer risk from a single CT scan (1-year AUC of 0.86–0.94). AI is also being used to phenotype patients and tumors at scale, analyzing digitized pathology slides to aid in tumor classification and predict mutational status.
Predicting Treatment Response and Toxicity with ai for real-world evidence
A key application of ai for real-world evidence is predicting treatment response and toxicity. By integrating disparate data sources like images, genomics, and pathology, AI can identify novel connections and significantly improve predictions beyond what single data types can achieve.
Federated learning, for instance, has successfully predicted histological response to chemotherapy in breast cancer. Radiomic analysis from CT images can non-invasively predict lung cancer EGFR genotype, aiding targeted therapy selection. AI can also dynamically update survival risks using longitudinal data and provide early warnings of treatment toxicities, improving patient safety.
Automating Clinical Data Extraction for ai for real-world evidence
Robust RWE requires comprehensive data. In oncology, critical information is often buried in unstructured clinical notes. Manual extraction is slow, error-prone, and unscalable.
AI, particularly NLP and LLMs, is indispensable for automating this process. Systems like DeepPhe extract cancer phenotypes from clinical records, while NLP pipelines pull key features like performance status and symptoms from notes. LLMs are proving highly effective for feature extraction and clinical prediction from free-text records. This automation accelerates research and ensures higher quality data for generating RWE.
The Reality Check: Overcoming Problems to AI and RWE Adoption
While the potential of ai for real-world evidence is transformative, we must be honest about the problems blocking its adoption. We face challenges with data quality, privacy, regulation, algorithmic bias, and clinical integration. Many impressive AI models exist, but we need more high-quality studies to prove their real-world benefit and to monitor their reliability and fairness over time.
The Data Challenge: Ensuring Quality, Security, and Interoperability
At its core, the challenge is the data itself. Real-world data is often messy, unstructured, and can carry unintentional biases from how it was collected or from underrepresented patient groups. For ai for real-world evidence to be reliable, data must be high-quality and standardized.
Furthermore, accessing and combining different data sources is tough due to strict privacy rules like GDPR and HIPAA and major interoperability issues between systems.
At Lifebit, our approach tackles these issues head-on. Automating data extraction improves privacy and security by reducing human handling. We use common data models like OMOP-CDM to standardize data, making it consistent across sources. We combine this with secure access protocols within a Trusted Data Lakehouse (TDL). This strategy ensures the data for RWE is high-quality, secure, and interoperable.
The Regulatory Maze: Building Trust and Gaining Approval
Navigating the regulatory landscape for ai for real-world evidence and AI-powered medical devices is another hurdle. Regulatory bodies like the FDA are actively working to shape this field, and our work must follow strict legal frameworks.
The FDA, for instance, has requested public input on evaluating the real-world performance of AI devices, as seen in their request for comment. This highlights the need for continuous monitoring of AI systems post-deployment to ensure they remain safe and effective. Strong validation methods, transparent processes, and constant monitoring are essential to ensure ai for real-world evidence is trustworthy and meets the highest safety standards.
The Human Factor: Integrating AI into Workflows and Ensuring Equity
The success of ai for real-world evidence hinges on smooth integration into clinical workflows and earning the trust of clinicians and patients. AI systems are still evolving and need rigorous validation in real clinical settings.
Building trust requires proactively addressing algorithmic bias and ensuring our models promote health equity. We must prioritize diversity in training datasets to prevent biases that could worsen health disparities. We also need Explainable AI (XAI) methods to make AI’s decisions transparent, which is vital for building confidence. Continuous monitoring for performance changes is key to maintaining trust and achieving fair outcomes in the ever-changing healthcare environment.
Frequently Asked Questions about AI for Real-World Evidence
We’ve explored how ai for real-world evidence is changing healthcare, but it’s natural to have questions. Here are some of the most common ones, answered simply.
What is the main benefit of using AI with RWE?
The main benefit of pairing AI with Real-World Evidence (RWE) is its ability to rapidly analyze vast, complex, and messy real-world datasets (RWD). Manually processing things like electronic health records and doctor’s notes is nearly impossible.
AI steps in to find hidden patterns and connections, generating actionable real-world evidence that would otherwise be missed. This accelerates research, personalizes patient care, and speeds up the entire drug development lifecycle by turning raw information into powerful insights.
Is AI-generated RWE accepted by regulatory bodies like the FDA?
Yes. Regulatory bodies like the FDA are not just open to using RWE—including AI-generated evidence—they are actively building frameworks to make it a core part of their decision-making. They recognize the value of understanding how treatments perform in the real world.
The key is ensuring trust and rigor. Regulators require that the data is high-quality, the analytical methods are robust and validated, and the entire process is transparent. This gives them confidence in the insights that ai for real-world evidence provides.
What are the biggest risks of implementing AI for RWE?
While the potential is huge, there are risks to manage. The most significant is poor data quality; the “garbage in, garbage out” principle means flawed data leads to flawed insights. Another major concern is algorithmic bias, where models trained on unrepresentative data can perpetuate health inequities.
Finally, data privacy and security are paramount, requiring robust safeguards to protect patient information. There’s also the hurdle of gaining trust from clinicians. If AI models aren’t transparent, explainable, and proven to be valuable, healthcare professionals will be reluctant to adopt them. Addressing these risks is crucial for successful and ethical implementation.
Conclusion: The Future of Healthcare is Real, and It’s Powered by AI
If one thing is clear, it’s this: ai for real-world evidence isn’t just an update to healthcare—it’s a complete rewrite of how we understand disease, develop treatments, and care for patients.
We’re seeing AI transform messy, fragmented data into life-saving insights. It’s slashing drug development timelines, helping oncologists predict treatment response, and empowering everyone from policymakers to patients to make smarter decisions. AI is already helping biopharma optimize trials, enabling providers to deliver value-based care, and giving oncologists a powerful new weapon against cancer.
However, the challenges are significant. Data quality, privacy, security, an evolving regulatory landscape, and building trust with clinicians are real problems that cannot be ignored.
This is exactly why we built Lifebit. Our federated AI platform addresses these challenges head-on. By providing secure, real-time access to global biomedical data, we enable insight generation without compromising patient privacy. Our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) deliver the benefits of AI-driven RWE while maintaining the rigorous governance healthcare demands.
We believe the future of healthcare must be intelligent, trustworthy, equitable, and collaborative. The change is already happening, with researchers generating insights that were impossible just years ago. And we’re just getting started.
If you’re ready to be part of this revolution, we’d love to show you what’s possible. Learn how to open up your real-world data with our R.E.A.L. solution and join us in building a future where every patient benefits from personalized, evidence-based care powered by ai for real-world evidence.
The future of healthcare is real. And it’s powered by AI.

