Smart Trials: How AI is Streamlining Clinical Research

Why Clinical Trials Need an AI Revolution
AI clinical trial optimization is changing how pharmaceutical companies and research institutions bring new therapies to patients. By leveraging machine learning, natural language processing, and predictive analytics, AI addresses the most critical bottlenecks in clinical research: slow patient recruitment, high operational costs, and lengthy development timelines.
Key ways AI optimizes clinical trials:
- Patient Recruitment: AI-powered tools improve enrollment rates by 65% and identify eligible candidates 3x faster by analyzing electronic health records
- Timeline Acceleration: AI integration accelerates trial timelines by 30-50% and reduces costs by up to 40%
- Protocol Optimization: Predictive analytics models achieve 85% accuracy in forecasting trial outcomes, helping prevent costly amendments
- Safety Monitoring: Digital biomarkers enable 90% sensitivity for adverse event detection through continuous monitoring
- Site Selection: AI analyzes historical performance data to identify high-yield sites and reduce overall site footprint
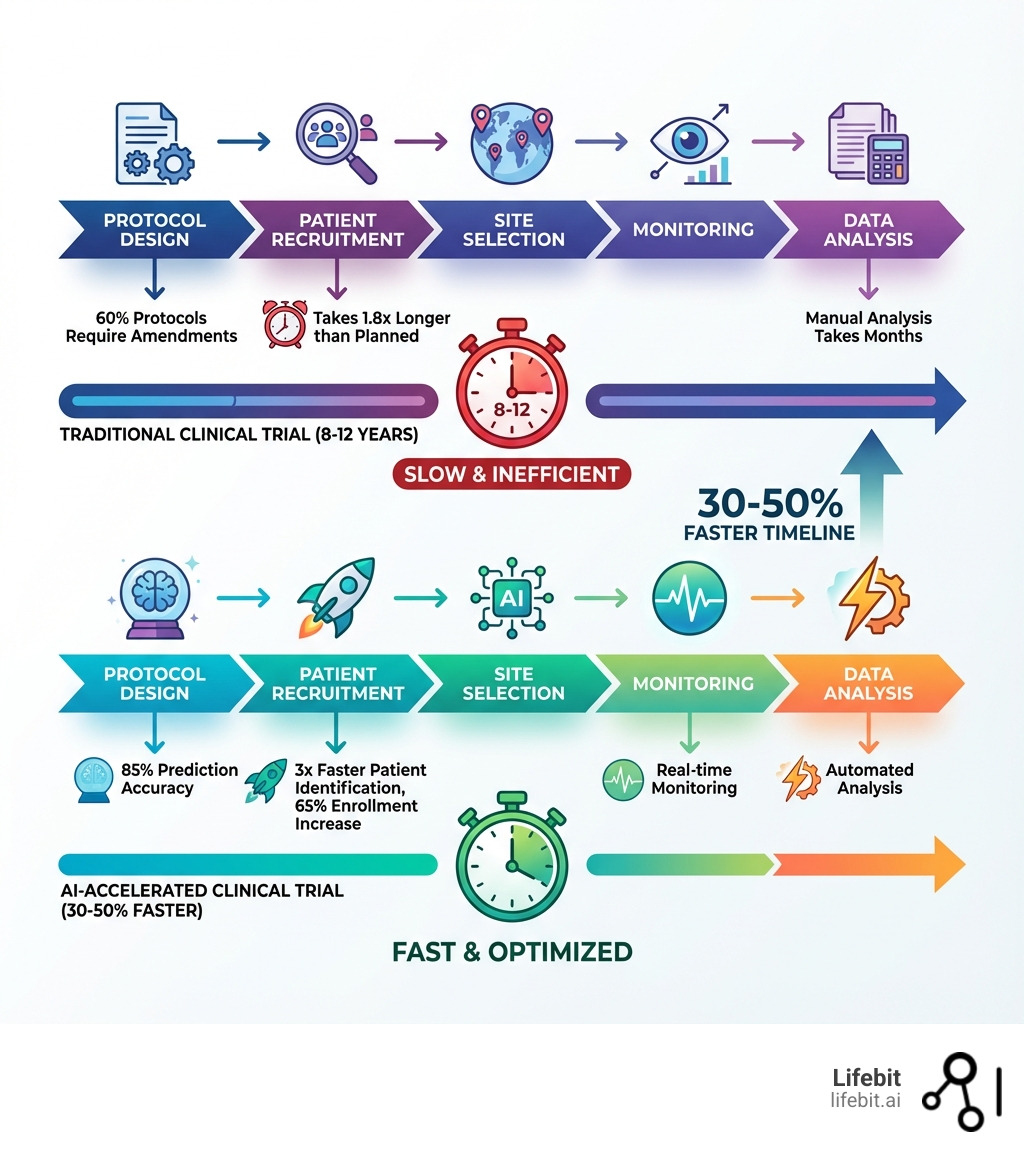
The stakes couldn’t be higher. Today, it takes over a billion dollars and up to 12 years to bring a new treatment to market. Clinical trials alone consume roughly 40% of total pharmaceutical research budgets. Meanwhile, 80% of trials miss enrollment deadlines, 60% require protocol amendments (half of which are avoidable), and only 10-15% of drugs entering clinical development ultimately receive approval.
Traditional clinical trial methods are buckling under mounting complexity. The number of procedures per trial has grown by 60% over the past decade. Each day a trial extends beyond its enrollment deadline translates to between $600,000 and $8 million in missed market opportunity. For patients waiting for breakthrough therapies, these delays can mean the difference between life and death.
AI offers a fundamentally different approach—one that transforms clinical research from a labor-intensive, error-prone process into an intelligent, adaptive system. From designing smarter protocols to identifying the right patients in minutes instead of months, AI is reshaping every stage of the clinical trial lifecycle.
As Maria Chatzou Dunford, CEO of Lifebit, I’ve spent over 15 years working at the intersection of computational biology, AI, and health-tech entrepreneurship, building platforms that enable ai clinical trial optimization through secure, federated data analysis. Our work with pharmaceutical companies and public sector institutions has shown that when you combine AI with the right data infrastructure, you can dramatically accelerate drug findy while maintaining rigorous compliance and patient privacy.
The Crisis in Clinical Trials: Why Traditional Methods Are Failing
Clinical trials are the bedrock of medical progress, the essential assessment for safe, reliable, and effective drug development. Yet, for decades, they have been plagued by systemic inefficiencies. It’s a complex, tangled process, often feeling like a race against time with one hand tied behind our backs.
One of the most vexing challenges is patient recruitment. An astonishing 80% of studies face recruitment delays, and nearly a third of all Phase III studies fail because of enrollment issues. It’s estimated that 86% of all trials don’t even meet their recruitment schedules. This isn’t just a minor inconvenience; patient enrollment typically takes 1.8 times longer than planned, leading to significant setbacks.
Beyond recruitment, the operational costs are astronomical. Pharmaceutical R&D expenditures exceed $200 billion annually, with clinical trials gobbling up roughly 40% of that budget. Why so much? The number of procedures per trial has grown by 60% over the past decade, making protocol design increasingly complex. These elaborate protocols and stringent eligibility criteria often narrow the patient pool, making recruitment even harder.
Then there are the amendments. Roughly 60% of trial protocols require at least one amendment, and nearly half of these are considered “avoidable.” These avoidable changes cost pharmaceutical companies an eye-watering $2 billion per year in direct costs and add an average of 260 days to development timelines. Imagine the impact on patients waiting for life-saving treatments!
Poor patient retention is another silent killer of trials. Close to 40% of patients discontinue their prescribed medication within the initial year of a clinical trial. This attrition compromises data integrity and extends timelines further. Data quality issues affect 50% of clinical trial datasets, exacerbating the problem.
Traditional methods also struggle with data management. Fragmented and unstructured data, often found in electronic health records (EHRs) as faxed records or handwritten notes, create significant interoperability challenges. This makes it difficult to extract meaningful insights efficiently.
The cumulative effect of these challenges is a staggering failure rate. Clinical trial success rates remain below 12%, and only one in seven drugs entering Phase I trials is eventually approved. This means immense efforts, time, and money are wasted, with potential losses from delayed product development and launch ranging from $600,000 to $8 million each day a trial extends beyond its deadline. Traditional clinical trials simply lack the analytical sophistication, flexibility, and speed needed for today’s complex novel medicines and diverse patient groups.
How AI Revolutionizes Every Stage of Clinical Research
The good news is that we are not helpless in the face of these challenges. AI clinical trial optimization offers transformative solutions to address these systemic inefficiencies across the entire clinical trial lifecycle. AI isn’t just a buzzword; it’s a suite of powerful technologies, including Machine Learning (ML), Deep Learning (DL), and Natural Language Processing (NLP), that can learn from vast datasets, identify patterns, and make predictions with unprecedented speed and accuracy.
Our mission is to harness AI for end-to-end optimization, turning clinical research from a reactive process into a proactive, data-driven one. AI for drug findy, for instance, uses machines to mimic human intelligence, solving complex problems in the drug development process. By adopting AI solutions, we can get rid of many potential problems, shorten trial timelines, and make the entire process more accurate and productive. This is why life science industry players are increasingly interested in these advanced AI solutions.
AI can analyze large datasets to identify patterns and make precise predictions, accelerating new treatment development. It simplifies procedures, decreases costs, and improves efficiency. Crucially, AI automates data generation and management throughout the trial lifecycle, including patient-centric AI. It can intelligently interpret data and feed downstream systems for automated analysis reports. This shift towards an AI-enabled approach is revolutionizing medical breakthroughs. Scientific research on AI in drug development highlights this potential.
Smarter by Design: AI-Powered Protocol and Cohort Selection
Imagine designing a trial that is inherently more likely to succeed. This is where AI-powered protocol and cohort selection shines. Predictive analytics models can achieve 85% accuracy in forecasting trial outcomes, helping us prevent costly amendments before they even happen. Instead of iterating through trial and error, we can simulate scenarios and optimize our approach.
AI allows us to sift through vast amounts of data, including electronic health records and social media, to identify suitable patient subsets and predict their responses. This is crucial for precision medicine, where identifying specific patient subgroups for targeted therapies can make all the difference. AI can also analyze the available data on an illness and compare it to the characteristics of a clinical study to generate optimal eligibility criteria. For instance, relaxing inclusion criteria using a data-driven approach has been shown to double the number of patients that would have been enrolled without compromising patient safety.
We can use AI for in-silico simulation, digitally testing drug effects and predicting outcomes before human trials even begin. This significantly reduces the need for protocol amendments, saving both time and money. AI-based clinical trial matching systems can achieve high accuracy in screening patients for trials, such as in oncology, by understanding complex protocols and patient data. This makes trials smarter by design, leading to higher success rates. Scientific research on AI for clinical trial design elaborates on these advancements.
Accelerating Enrollment: A Core Goal of AI Clinical Trial Optimization
Patient recruitment is often the biggest bottleneck in clinical trials, but AI is changing this dramatically. AI clinical trial optimization tools are improving enrollment rates by 65%. How? By leveraging Natural Language Processing (NLP) to analyze unstructured data from EHRs, such as clinical notes and charts. This allows for automated patient-trial matching that identifies eligible candidates three times faster than manual review, with some systems achieving 96% accuracy.
Consider the impact: patient recruitment cycles that used to span months are now shrinking to days. Study builds that took days now take minutes. For example, certain AI systems have led to a 24%-50% increase in accurately identified eligible patients, taking minutes for eligibility screening compared to the weeks or months required for standard prescreening in cancer trials. One platform demonstrated a 170x speed improvement at a major clinic, enabling faster enrollment across oncology, cardiology, and neurology trials.
AI also improves personalized patient engagement. It can analyze social media and patient support groups to detect illness clusters, helping us identify potential participants more efficiently and streamline complex admissions requirements. The use of AI-powered clinical trial matching systems has already facilitated increased enrollment and awareness of clinical trial opportunities. Automated EHR text-mining for cardiovascular trials can result in a 79.9% reduction in the number of patients needing to be screened.
While AI is primarily applied to recruitment, we acknowledge a significant gap in its application for participant retention. This is an area ripe for future development. However, the current benefits are clear: increased efficiency, cost savings, improved recruitment rates, improved accuracy, and better patient satisfaction through user-friendly interfaces. A scoping review on AI for recruitment and retention provides a comprehensive overview of these opportunities.
Optimizing Site Selection and Real-Time Monitoring
Finding the right trial sites is crucial for success. AI helps us identify high-performing sites by analyzing historical performance, capacity, and recruitment rates. This allows for more accurate predictions of enrollment rates and smarter resource allocation. AI-driven tools, such as those developed by leading biopharma companies, analyze large structured and unstructured datasets to identify sites most likely to meet participant enrollment goals, optimizing trial design and reducing duration. This also enables diversity targeting, helping us find high-potential sites with access to underrepresented populations, promoting equitable trial participation.
Once a trial is underway, continuous, real-time monitoring is paramount. This is where digital biomarkers and wearable technology truly shine. Digital biomarkers enable 90% sensitivity for adverse event detection. Imagine a wearable ECG device using ML on real-time data to identify irregular cardiac rhythms such as atrial fibrillation. This allows for proactive risk mitigation, catching potential issues before they escalate.
AI-powered voice assistants are already being used in many clinical trials for routine monitoring tasks, from reminding patients about appointments to tracking daily activities. These digital health technologies facilitate longitudinal and real-time biometric data collection, shedding light on the lasting effects of medicines and treatment procedures in the real world. This continuous, patient-centric surveillance system, powered by AI, ensures greater safety and allows for adaptive interventions, making trials more responsive and ultimately, more successful.

The Next Frontier: AI Clinical Trial Optimization with Digital Twins
If AI is the engine, then digital twins are the vehicle driving us into the future of clinical research. Digital twins, or virtual patient populations, are AI-generated models that simulate human patients. This allows drug candidates to be tested virtually, reducing the reliance on human subjects and accelerating drug development.
Imagine creating a dynamic virtual representation of an individual patient or an entire subgroup. These digital twins integrate real-world data, disease biology, pathophysiology, and known pharmacology into a single computational framework. They can simulate patient responses to treatments, allowing us to:
- Test drug candidates virtually before moving into the clinic.
- Assess safety and efficacy with potentially better accuracy in early phases.
- Determine optimal dosing virtually, potentially allowing a direct move from Phase 1 to Phase 2b studies.
- Supplement or even replace human clinical trials in rare diseases where patient recruitment is difficult or impossible.
- Create external control arms, reducing the need for placebo groups and making trials more patient-centric. Specialized software already uses AI and digital twins to achieve this, improving trial success rates with fewer patients.
These virtual patient models serve as the “scientific memory” of a disease, constantly updated with data from ongoing trials. Digital twin simulations applied to Alzheimer’s datasets, for example, have shown alignment with historical patient trajectories, helping to assess surrogate endpoints and trial enrichment strategies. Projects like TWIN-GPT are demonstrating the ability to impute missing values and synthesize patient trajectories from sparse datasets.
The ultimate goal for us is to build a “biology foundation model” trained on diverse data to predict clinical efficacy across drug development. This future involves building algorithms for deep learning to anticipate unknowns in complex diseases, expanding the applicability of virtual patients beyond well-understood single-gene mutations. This represents a profound shift, moving us closer to personalized medicine.
Navigating the Problems: Challenges, Ethics, and Regulation
While the promise of AI clinical trial optimization is immense, we must approach its implementation with open eyes, acknowledging the significant problems that lie ahead. The journey isn’t without its bumps, and navigating these challenges responsibly is key to open uping AI’s full potential.
Ethical and Technical Challenges in AI Clinical Trial Optimization
The path to integrating AI into clinical trials is paved with both technical and ethical considerations. On the technical front, data remains a primary challenge. We face issues like data quality, interoperability, and the sheer fragmentation of electronic health records (EHRs). Imagine trying to build a perfect AI model when half your datasets have quality issues, or when patient records come in as faxes, PDFs, or even handwritten notes. This unstructured and siloed data makes it incredibly difficult to train robust AI systems. Integrating AI with legacy systems, which are common in many healthcare institutions, also presents a significant technical barrier.
Then there’s the “black box” problem. Many advanced AI algorithms, especially deep learning models, can make highly accurate predictions but struggle to explain how they arrived at those conclusions. This lack of clear reasoning can lead to issues of blind trust and makes model validation difficult, particularly in safety-critical domains like clinical trials. Limited generalizability and insufficient external validation data are also concerns; an AI model trained on one population or institution may not perform as well when applied to others, potentially introducing unexpected biases.
Ethically, the stakes are even higher. Patient privacy and data security are paramount. With AI systems processing vast amounts of sensitive health information, ensuring robust data protection and informed consent models is critical. There are also concerns around data ownership and control.
Perhaps one of the most pressing ethical challenges is algorithmic bias. AI models trained on datasets that historically underrepresent certain demographics (e.g., patients of European and Caucasian descent) can perpetuate or even exacerbate existing biases. This can lead to skewed enrollment, discriminatory outcomes, and unequal access to potentially life-saving treatments. We must be vigilant in designing AI systems that are fair and equitable, ensuring they don’t leave anyone behind. The complexity of these algorithms also means there’s a need for universal guidelines and rigorous external validation.
At Lifebit, we understand these challenges intimately. Our federated AI platform is designed precisely to address data interoperability, security, and bias. By enabling secure, real-time access to global biomedical and multi-omic data, we can harmonize diverse datasets and apply advanced AI/ML analytics. Our built-in federated governance ensures data remains protected within its source, mitigating privacy and security risks. Components like our Trusted Research Environment (TRE) and Trusted Data Lakehouse (TDL) provide the infrastructure for compliant research, allowing us to deliver real-time insights and AI-driven safety surveillance across hybrid data ecosystems, all while fostering secure collaboration.
The Regulatory Landscape and Promoting Equity with AI
The regulatory landscape for AI in clinical trials is still evolving. Currently, there isn’t one standardized framework, which creates uncertainty for developers and researchers. However, bodies like the European Commission are stepping up, with proposals like the Artificial Intelligence Act (AIA) aiming to ensure AI is safe, lawful, and aligned with fundamental rights. High-risk AI systems, particularly those in healthcare, will need to meet mandatory requirements for trustworthy AI and undergo conformity assessments. In the United States, the CURES Act grants patients access to their medical records, highlighting a broader trend towards data transparency.
We believe that future frameworks will need to provide further guidance and standard-setting regarding transparency, oversight, and data quality in AI-ML processes. This includes developing clear validation standards and reporting guidelines, such as CONSORT-AI and DECIDE-AI, for AI-related trials. Collaborative regulatory sandboxes will be essential for trialing new AI frameworks under oversight.
Promoting diversity and equity in clinical trial participation is another critical area where AI can make a difference, provided we address its inherent biases. Companies must include health equity considerations in their AI and ML systems. This means training models on diverse and comprehensive datasets to mitigate biases and ensure equitable healthcare outcomes. Inclusive policies, alongside AI solutions, are needed to address disparities in healthcare access.
We advocate for bringing domain experts, such as medical professionals, into the algorithm development process to fill crucial context gaps. Patients and caregivers should also be included to ensure the real-world ramifications of algorithmic decisions are understood and addressed. Organizations developing AI for clinical trials must establish procedures for regularly assessing and correcting any biases. Our federated approach, which allows for training models across diverse, geographically distributed datasets without centralizing sensitive patient information, is a powerful tool for building more equitable and robust AI models.
Frequently Asked Questions about AI in Clinical Trials
How does AI speed up clinical trials?
AI speeds up clinical trials by automating many time-consuming, manual tasks and optimizing complex processes. For example, AI-powered tools can identify eligible patients from vast electronic health records three times faster than traditional methods, shrinking recruitment cycles from months to days. AI also optimizes trial design, using predictive analytics to forecast outcomes with 85% accuracy, which helps prevent costly protocol amendments that can add 260 days to a trial. Furthermore, AI accelerates data analysis, extracting insights from large, multi-modal datasets in real-time, leading to overall timeline reductions of 30-50%.
Is AI replacing human researchers in clinical trials?
Absolutely not! AI is an augmentation tool, not a replacement. It empowers human researchers by taking on the heavy lifting of data-intensive tasks, such as sifting through millions of patient records for eligibility, monitoring vast streams of real-time data from wearables, or running complex simulations. This frees up human experts to focus on what they do best: strategic decision-making, direct patient care, interpreting scientific results, and applying their invaluable clinical judgment. AI helps bridge the gap where humans are incapable of multitasking across massive datasets, allowing researchers to be more efficient and innovative.
What is the biggest challenge to implementing AI in clinical trials?
The primary challenge to implementing AI in clinical trials is accessing high-quality, interoperable data. Clinical data is often fragmented across different systems, unstructured (think handwritten notes or faxes), and stored in silos. This makes it incredibly difficult to build robust AI models that can learn and make accurate predictions. Furthermore, privacy regulations (like GDPR in Europe) and institutional policies often restrict data sharing, hindering the creation of the large, diverse datasets needed for effective AI training. Advanced solutions like federated learning are crucial here, allowing AI models to learn from decentralized data sources without the data ever leaving its secure environment, preserving privacy and promoting collaboration.
Conclusion
The journey of drug development is arduous, expensive, and fraught with uncertainty. But the era of AI clinical trial optimization is upon us, offering a guide of hope for faster drug findy, cheaper development costs, and more efficient trials. We are moving towards a future where AI is not just an auxiliary tool, but a standard, indispensable component of clinical research.
The benefits are clear and quantifiable: AI can accelerate trial timelines by 30-50%, reduce costs by up to 40%, boost patient recruitment rates by 65%, and enable real-time safety monitoring with 90% sensitivity for adverse events. From designing smarter protocols with 85% prediction accuracy to leveraging digital twins for in-silico trials, AI is changing every stage. This isn’t just about efficiency; it’s about making medicine more patient-centric, bringing breakthrough therapies to those who need them most, sooner.
The path to personalized medicine, where treatments are custom to individual patients, is paved with AI. However, realizing this future demands that we proactively address the technical and ethical challenges, build robust data infrastructures, and foster collaborative regulatory frameworks.
At Lifebit, we are at the forefront of this revolution. Our federated AI platform is purpose-built to tackle the complexities of clinical data, enabling secure, real-time access to global biomedical and multi-omic data. With built-in capabilities for harmonization, advanced AI/ML analytics, and federated governance, we empower biopharma, governments, and public health agencies in Europe, the USA, Canada, the UK, Israel, Singapore, and beyond, to conduct large-scale, compliant research. Our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) deliver real-time insights, AI-driven safety surveillance, and secure collaboration across hybrid data ecosystems.
The future of clinical research is intelligent, efficient, and equitable. We are building it, one smart trial at a time.
Discover how a federated platform can accelerate your research


