AI-Powered Biomarker Discovery: Enhancing Precision in Cancer and Beyond

Why AI-Powered Biomarker Findy Is Changing Cancer Treatment
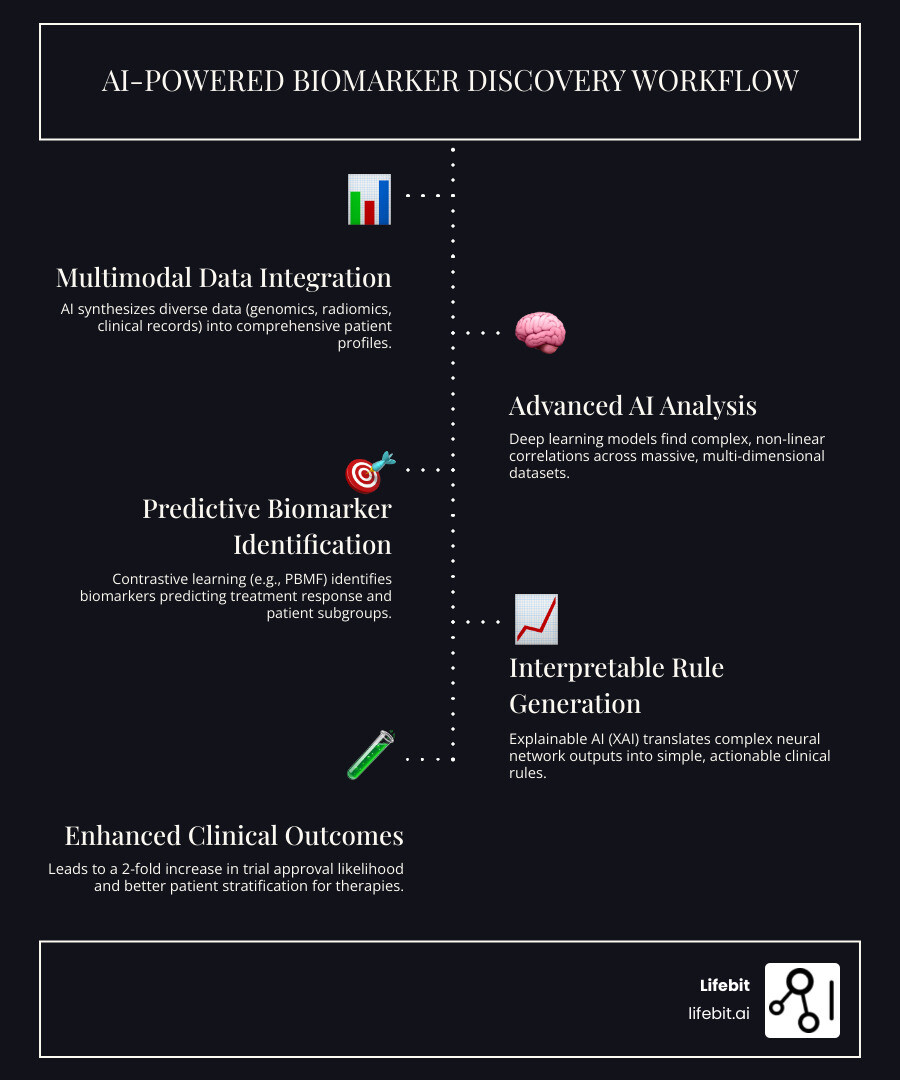
AI-powered biomarker findy uses artificial intelligence to analyze complex biological data—genomics, imaging, and clinical records—to find biomarkers that predict patient response to specific treatments. This directly tackles a critical industry failure: 90% of therapies in development fail to reach the market, largely because they aren’t matched to the right patients.
Unlike traditional methods that struggle with the sheer volume and complexity of modern biological data, AI thrives on it. Deep learning models find complex, non-linear patterns across massive datasets that older statistical models miss entirely. This allows for:
- True Predictive Power: Identifying patients who will benefit from a specific therapy, not just forecasting general outcomes.
- Multimodal Integration: Synthesizing genomics, radiomics, and clinical data into a comprehensive patient view.
- Interpretable Insights: Translating complex AI models into simple decision trees that clinicians can trust and apply.
The impact is already clear. Drug development programs using biomarker preselection see their approval likelihood double from 8% to 16%. The field is exploding, with 80% of recent studies on AI in immunotherapy published in just 2021-2022.
AI can predict molecular biomarkers from routine pathology slides with up to 97% accuracy and integrate imaging with genomics to find patient subgroups with dramatically better treatment responses. As CEO and Co-founder of Lifebit, my work in computational biology and AI has provided a front-row seat to this change. Our pioneering work in federated data analysis is built to solve these challenges, enabling ai-powered biomarker findy across secure, distributed healthcare datasets.
The Crisis in Biomarker Findy: Why Traditional Methods Are Hitting a Wall
Personalized medicine relies on biomarkers, but traditional findy methods are buckling under the weight of modern biology. The problem is twofold: biological complexity and data overload.
First, cancer is incredibly complex. A single tumor contains diverse, evolving cell populations (tumor heterogeneity) within a dynamic microenvironment of immune cells, blood vessels, and signaling molecules. This intricate system means two seemingly identical cancers can be fundamentally different at a molecular level. Traditional methods, which focus on single gene mutations or protein markers, cannot capture this complexity. It’s challenging to identify new biomarker candidates when the biological target is constantly changing.

Second, modern clinical trials generate tens of thousands of measurements per patient—from genomics to medical images. This data should be a goldmine, but traditional statistical tools like Cox proportional hazards models are drowning. Designed for simpler, linear relationships, they cannot process the non-linear, multi-dimensional patterns hidden in today’s datasets. This is especially true when hunting for predictive biomarkers (who will benefit from a specific treatment) versus prognostic ones (who has a poor outlook regardless of treatment).
The slow, manual, hypothesis-driven approach to validation only makes things worse. The tools that brought us this far were not designed for the journey ahead. A new paradigm is needed to turn data overload into actionable insights—and that’s where AI enters the picture.
A New Paradigm: How AI-Powered Biomarker Findy Works
The limitations of traditional methods have paved the way for AI-powered biomarker findy. Instead of relying on educated guesses, AI sifts through massive datasets to uncover patterns invisible to conventional statistics.
The Power of Contrastive Learning in Identifying Patient Subgroups
At the heart of this shift is the Predictive Biomarker Modeling Framework (PBMF), which uses a clever technique called contrastive learning. The goal is to find patients who benefit specifically because of a treatment, not just those with a better prognosis.
PBMF’s neural networks do two things at once: they maximize the survival difference between responders and non-responders in the treatment group while minimizing any survival difference in the control group. This isolates the treatment effect, allowing the model to distinguish true predictive biomarkers from merely prognostic ones. Unlike traditional methods that struggle with complexity, neural networks thrive on it, analyzing thousands of measurements per patient to find the intricate, non-linear relationships that determine treatment response.
From AI ‘Black Box’ to Actionable Clinical Rules
A major barrier to AI adoption is the “black box” problem—powerful models that are too opaque for clinicians to trust. Explainable AI (XAI) is the solution.
PBMF uses model distillation to translate its complex neural network into a simple, interpretable decision tree. This process is like turning a dense, intricate map into a clear set of directions. The result is an actionable clinical rule that a doctor can understand, trust, and apply. For example, a rule might state that patients with a specific gene mutation and protein level are highly likely to respond to immunotherapy. This step is critical for bridging the gap between cutting-edge AI and real-world medical practice.
How the PBMF Compares to Existing Methods
PBMF represents a significant leap forward, but how does it stack up against other methods?
| Feature | PBMF (Predictive Biomarker Modeling Framework) | SIDES (Subgroup Identification based Differential Effect Search) | Virtual Twins (VT) | Traditional Statistical Models (e.g., Cox PH) |
|---|---|---|---|---|
| Primary Goal | Predictive biomarker findy | Subgroup identification | Subgroup identification | Prognosis/association |
| Methodology | Neural network with contrastive learning | Recursive partitioning/decision trees | Ensemble of decision trees (random forests) | Regression analysis |
| Predictive Focus | Directly optimizes for treatment-specific benefit | Focuses on differential treatment effect in subgroups | Estimates individual treatment effects | General outcome prediction (can be adapted) |
| Non-linear Relationships | Excellent | Good | Good | Limited (requires manual interaction terms) |
| Data Type Handling | Highly diverse (multi-omics, clinical) | Diverse (structured data) | Diverse (structured data) | Primarily structured numerical data |
| Interpretability | High (via knowledge distillation to decision trees) | High (tree-based) | Moderate (ensemble interpretation can be complex) | High (coefficient-based) |
| Prognostic vs. Predictive | Explicitly distinguishes via contrastive loss | Can sometimes conflate if not carefully designed | Can sometimes conflate | Needs careful interpretation/interaction terms |
| Robustness | High (with ensemble and pruning) | Moderate | High | Moderate |
PBMF’s unique contrastive learning objective is what sets it apart. It is specifically engineered to isolate treatment-specific benefits, whereas other AI methods like SIDES and Virtual Twins can inadvertently identify prognostic markers. In simulated scenarios, PBMF achieved an AUPRC (Area Under the Precision-Recall Curve) of 0.918 ± 0.047, significantly outperforming Virtual Twins at 0.858 ± 0.029. This demonstrates its superior ability to find true predictive biomarkers in complex, noisy, real-world data.
Open uping the Power of Multimodal Data with AI
Traditional biomarker findy often fails by looking at just one piece of the puzzle. Cancer doesn’t work in silos, and neither should our analysis. The human body is a complex system where genes, proteins, and cellular environments all interact. AI-powered biomarker findy excels because it can analyze the entire system at once.
This is the power of multimodal data integration. Instead of analyzing genomics (DNA/RNA), radiomics (imaging features), proteomics (protein analysis), and clinical data (EHRs) separately, AI synthesizes them into a single, powerful model.
AI frameworks can spot connections across data types that are invisible to human researchers. Studies on AI for multimodal data integration in oncology show that combining radiology, pathology, and genomics can predict immunotherapy response with remarkable accuracy and improve risk stratification in ovarian cancer.
At Lifebit, our federated AI platform is designed for this complexity. Our Trusted Data Lakehouse (TDL) harmonizes diverse data streams, while our R.E.A.L. (Real-time Evidence & Analytics Layer) enables real-time analysis across distributed datasets—all while maintaining strict data privacy and governance.
Another exciting aspect is transfer learning. An AI model can be pretrained on millions of general biological samples and then fine-tuned on specific cancer data. This “educated” model adapts quickly, reducing the need for large, specialized datasets—a huge advantage in rare diseases. By combining a patient’s genetic makeup, tumor images, protein profiles, and clinical history, we create a detailed portrait of their disease that mirrors biological reality.
Real-World Impact: From Retrospective Analysis to Future Clinical Practice
The true test of any breakthrough is its real-world impact. Here, ai-powered biomarker findy is moving from promising theory to tangible results.
Rescuing Clinical Trials and Refining Patient Selection
A staggering 90% of therapies in development never reach market approval, often because they are tested on the wrong patients. Integrating patient preselection biomarkers can double the approval odds from 8% to 16%. In an industry where one approved drug saves thousands of lives, this is transformative.
Retrospective analysis using the PBMF framework has already shown exciting results. In one phase 3 trial, it identified a predictive biomarker that led to a 15% improvement in survival risk compared to the original trial population. Similar improvements have been found in other studies, revealing clear responders who were previously lost in the statistical noise. However, retrospective success isn’t enough. The crucial next step is validation in prospective trials designed from the ground up to test these AI-identified biomarkers.
Beyond Survival: New Frontiers for AI-Powered Biomarkers
Survival is critical, but AI’s potential extends further:
- Adverse Event Prediction: Analyzing multi-modal data to identify patients at high risk for severe side effects, allowing for proactive risk management.
- Dosing Optimization: Tailoring drug doses to an individual’s unique biological profile to maximize benefit and minimize toxicity.
- Companion Diagnostics: Accelerating the development of tests that identify which patients will benefit from a specific drug.
The same principles apply beyond oncology to autoimmune disorders, neurological conditions, and infectious diseases, laying the foundation for personalized medicine across the entire healthcare spectrum.
Navigating the Challenges of Real-World Implementation
Despite the promise, significant obstacles remain. Data quality and bias are chief among them; AI models are only as good as their training data. Most models are trained on limited, non-diverse populations, which can worsen health disparities. Addressing bias and privacy concerns in cancer diagnostic data is fundamental to ensuring equitable care.
Furthermore, using sensitive patient data requires robust governance and privacy-enhancing technologies. This is where Lifebit’s federated AI platform makes a critical difference, enabling secure analysis of multimodal data without moving it from its source. Finally, the regulatory landscape for AI-based biomarkers is still evolving. Overcoming these challenges requires close collaboration between AI researchers, clinicians, regulators, and industry partners to ensure this technology is deployed ethically, equitably, and effectively.

Frequently Asked Questions about AI in Biomarker Findy
What is the difference between a predictive and a prognostic biomarker?
A prognostic biomarker forecasts a disease’s likely progression, regardless of treatment. It tells you a storm is coming but not if an umbrella will help. For example, it might indicate an aggressive cancer with a poor prognosis.
A predictive biomarker is a personalized treatment guide. It identifies whether a patient will likely benefit from a specific therapy. This is the key to precision medicine, ensuring patients get treatments custom to their unique biology. AI-powered biomarker findy focuses on finding these actionable, predictive markers.
Why is Explainable AI (XAI) so critical for clinical adoption?
Clinicians cannot base life-or-death decisions on a “black box” algorithm. They need to understand why an AI model makes a recommendation. Explainable AI (XAI) provides this transparency, building the trust necessary for clinical adoption.
XAI allows clinicians to validate that the AI’s findings make biological sense and helps identify potential biases in the training data. Techniques like model distillation, which turns complex neural networks into simple decision trees, create clinically applicable rules that doctors can understand and use confidently. This interpretability is essential for moving AI from the research lab to the bedside.
How does AI handle complex data better than traditional statistical methods?
Traditional statistical methods like Cox models were designed for a simpler data era and struggle with the scale and complexity of modern biomedical data. AI’s advantages are threefold:
- Handling Non-Linearity: Biological systems are not linear. AI models like neural networks automatically learn complex, non-linear relationships without researchers having to guess which variables interact.
- Managing High Dimensionality: Modern datasets contain tens of thousands of measurements per patient. AI is built to process this high-dimensional data directly, where traditional methods would buckle.
- Multimodal Integration: AI can simultaneously learn from genomics, imaging, and clinical records, synthesizing them into a holistic patient profile. This uncovers connections between different data types that older methods would miss.
Conclusion: The Dawn of AI-Driven Precision Oncology
The shift from a 90% clinical trial failure rate to truly personalized cancer care is happening now, driven by AI-powered biomarker findy. This technology is fundamentally reshaping how we match patients to effective treatments.
AI is overcoming decades-old challenges like tumor heterogeneity and data overload. Through techniques like contrastive learning and multimodal data integration, AI finds the true predictive signals that determine treatment success. By making these models explainable, we ensure clinicians can trust and apply these insights at the bedside.
The results are compelling: a 2-fold increase in approval likelihood for drugs developed with AI-identified biomarkers and 15% improvements in survival risk when patients are correctly matched to treatments. The applications are expanding beyond survival prediction to include adverse event prediction, dose optimization, and companion diagnostic development.
Significant challenges in data quality, bias, privacy, and regulation remain. The path forward requires collaboration and robust technological infrastructure. This is why Lifebit built its federated AI platform. Our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) enable secure, real-time analysis of multimodal data without compromising privacy or governance. We are building the infrastructure to make ethical, equitable precision medicine possible at scale.
The era of AI-driven precision oncology is here. Better patient outcomes are no longer an aspiration—they are becoming the standard.

Power your biomarker research with advanced data science.
For more information on how our federated AI platform can accelerate your research and development efforts, contact us today.

