Clinical Trial Data Made Simple (No Lab Coat Required!)
Clinical trial data: Master 2025’s Simple Access
Why Clinical Trial Data Matters More Than Ever
Clinical trial data is the backbone of medical progress, detailing research on new treatments, drugs, and medical devices. It covers study protocols, eligibility criteria, safety results, and outcomes, helping researchers, patients, and providers make informed decisions.
Quick Access Guide to Clinical Trial Data:
- Primary Global Database: ClinicalTrials.gov (300,000+ studies worldwide)
- Key Data Elements: Study protocols, eligibility criteria, outcome measures, adverse events
- Major International Registries: WHO ICTRP, EU Clinical Trials Register, ISRCTN
- Patient Privacy: Protected through anonymization and de-identification
- Data Access: Most registries offer free public access with search capabilities
- Study Phases: Phase 1 (safety), Phase 2 (effectiveness), Phase 3 (large-scale), Phase 4 (post-marketing)
The push for transparency has transformed access to this information. As one researcher noted about the AACT database: “AACT is a publicly available relational database that contains all information about every study registered in ClinicalTrials.gov.” Now, data from over 80 million patient lives is accessible to researchers worldwide, powering drug findy and treatment optimization.
However, navigating this landscape is challenging. Data is often siloed, quality varies, and researchers face slow onboarding and regulatory bottlenecks, limiting real-time insights.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. For over 15 years, I’ve focused on changing how organizations access and analyze biomedical data, including clinical trial data, in secure, federated environments. My work breaks down data silos to enable real-time analytics while ensuring patient privacy and regulatory compliance.
What’s Inside a Clinical Trial Record?
Think of clinical trial data as the DNA of medical research. These detailed records capture a study’s entire journey, from vision to conclusion, helping you steer medical research with confidence.
Key Components of a Clinical Trial Data Record
Every trial begins with a protocol, the master plan guiding the study. This comprehensive document goes beyond simple goals, detailing the scientific rationale, methodology, statistical considerations, and organization of the trial. It includes the Statistical Analysis Plan (SAP), which pre-specifies how the data will be handled and analyzed to prevent bias. The protocol also defines the data to be collected in Case Report Forms (CRFs). Each trial is assigned a unique NCT Number (e.g., NCT01234567) for tracking across global databases, and its study status (e.g., Not yet recruiting, Recruiting, Active, Completed, Terminated) provides a real-time snapshot of its progress.
The eligibility criteria are crucial safety and efficacy guidelines. Inclusion criteria define the target participants (e.g., adults aged 18-65 with a confirmed diagnosis of moderate-to-severe rheumatoid arthritis who have failed at least one TNF inhibitor). Exclusion criteria identify factors that would make participation unsafe or confound the results (e.g., presence of active infection, severe kidney or liver impairment, or concurrent use of other specific medications). These criteria ensure the study population is homogenous enough to test the hypothesis while being representative of the intended patient group.
The outcome measures define the study’s goals and are the metrics used to evaluate the intervention’s effects. Primary outcomes are the most important questions the trial is designed to answer (e.g., does a drug shrink tumors by a specific percentage, known as the objective response rate?). Secondary outcomes measure additional effects that are of interest but not the main focus (e.g., improved quality of life, reduction in pain, or overall survival). Other types of endpoints include surrogate endpoints (e.g., lowering cholesterol as a proxy for preventing heart attacks) and patient-reported outcomes (PROs), which capture the patient’s own assessment of their health. Adverse events (AEs), any untoward medical occurrence in a participant, are meticulously documented. These are further classified into Serious Adverse Events (SAEs)—events that are life-threatening, require hospitalization, or result in disability—to provide a complete picture of a treatment’s risk-benefit profile. An independent Data and Safety Monitoring Board (DSMB) often reviews this accumulating safety data to protect participants.
Understanding the Phases of Clinical Trials
Clinical trials unfold in phases, with the clinical trial data from each stage answering different questions. This phased approach is designed to maximize safety and ensure scientific rigor.
Phase 0 (Exploratory Studies): These are very small, first-in-human trials that give a preliminary look at how a drug behaves in the body. They involve very low, sub-therapeutic doses to gather data on pharmacokinetics (what the body does to the drug) and pharmacodynamics (what the drug does to the body) without risking toxicity.
Phase 1 tests safety and dosage in a small group (20-100) of healthy volunteers or, in some cases like oncology, patients with the disease. The goal is to determine a safe dosage range and identify side effects. Common designs include Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) studies.
Phase 2 tests effectiveness and further evaluates safety in a larger group (100-300) of patient groups with the condition. This phase is often split into Phase 2a (proof-of-concept studies to see if the drug has any biological activity) and Phase 2b (dose-ranging studies to find the optimal dose for the next phase). It tracks safety and early signs of efficacy to see if the treatment warrants further study.
Phase 3 involves large-scale testing (hundreds to thousands of participants) to confirm effectiveness, monitor side effects, and compare the new treatment to standard care. These are often randomized, double-blind, controlled trials—the gold standard for clinical evidence. Comprehensive data on effectiveness and safety is collected to support regulatory approval from bodies like the FDA or EMA.
Phase 4 is post-marketing surveillance conducted after a treatment is approved and on the market. These studies monitor long-term safety and effectiveness in a broad, diverse population, often using real-world evidence. They can identify rare side effects not seen in smaller trials and may even uncover new potential uses for the treatment.
Each phase generates robust clinical trial data that helps us understand if, how, and for whom treatments work. For help with terminology, the Glossary of Clinical Trial Terms provides clear definitions.
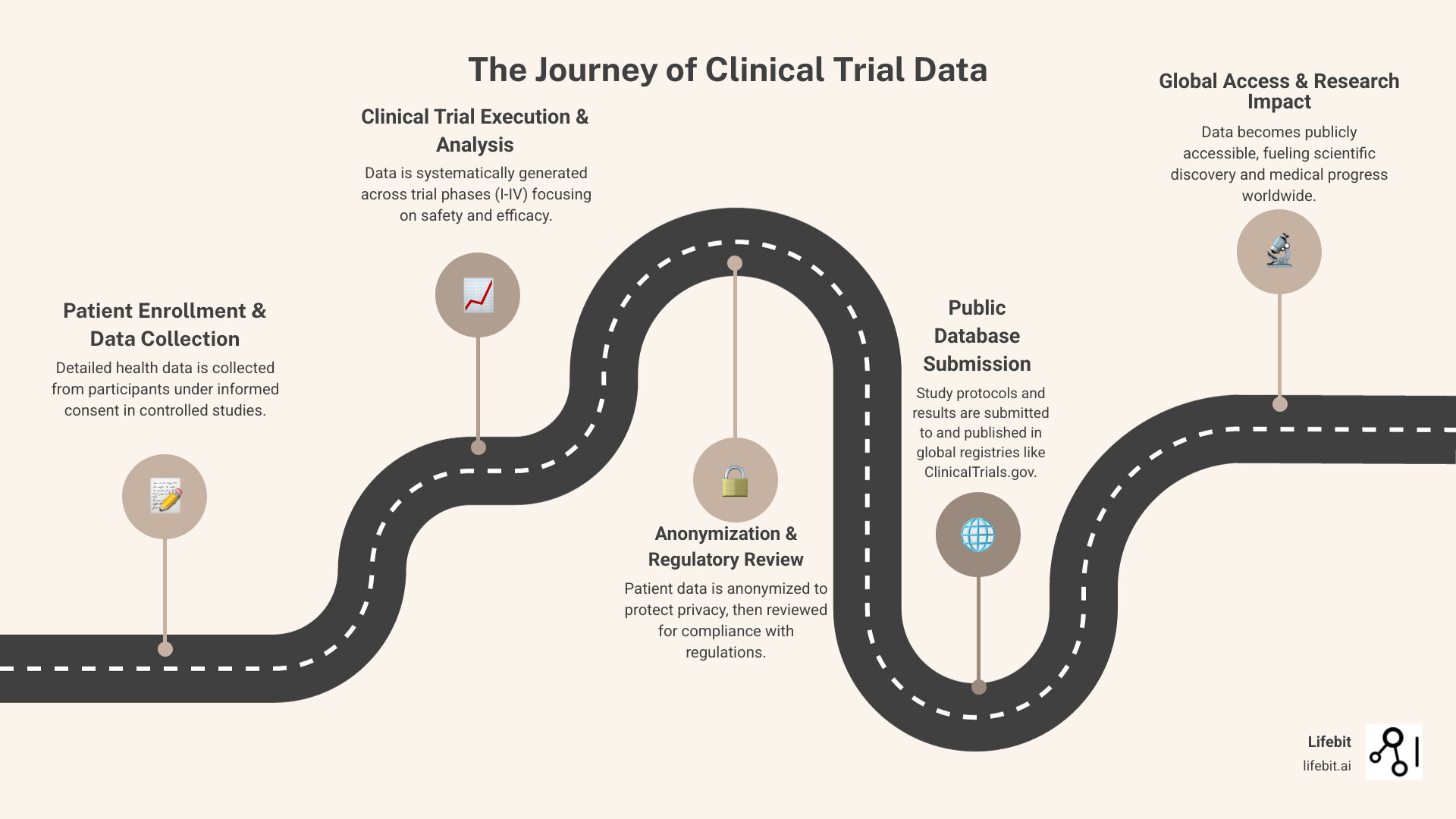
Your Global Map to Clinical Trial Data
Clinical trial data is stored in registries worldwide, forming a vast, interconnected network. Global collaboration has created a digital map connecting these repositories, making information accessible to researchers, patients, and providers. These living databases are constantly updated, serving as a gateway to global clinical research.
Navigating ClinicalTrials.gov
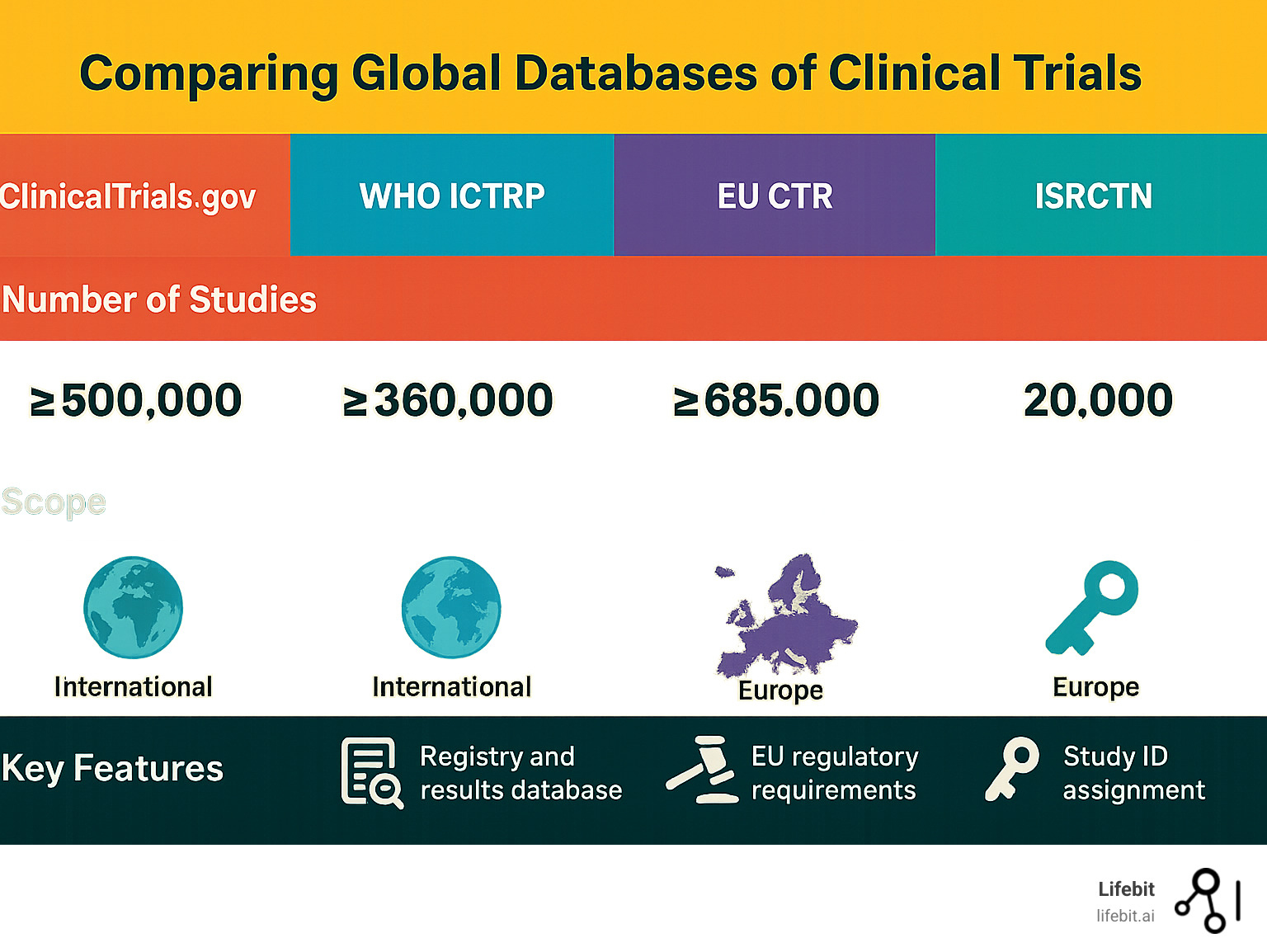
ClinicalTrials.gov, maintained by the U.S. National Library of Medicine (NLM) at the National Institutes of Health, is the world’s largest and most influential registry for clinical trial data. Its creation was mandated by law, and its scope was significantly expanded by the FDA Amendments Act of 2007 (FDAAA 801), which requires registration and results reporting for most trials. It hosts over 300,000 studies from more than 200 countries. Its value lies in its structured, user-friendly search; you can search by condition, intervention, or location and find a trial’s study purpose, design, and participation criteria. The system empowers patients and families to find new treatments, with clear guidance on How to Use Search on ClinicalTrials.gov. Despite its utility, searching can be challenging due to inconsistent terminology used by different sponsors. Modern technology is also improving digital clinical trial recruitment, making participation more accessible.
Exploring Major International Registries
Beyond ClinicalTrials.gov, a constellation of international registries enriches the global landscape of clinical trial data.
The WHO International Clinical Trials Registry Platform (ICTRP) acts as a central search portal, providing a single point of access to trial information from numerous registries worldwide. It aggregates data from primary registries, ensuring that a single search on the WHO International Clinical Trials Registry Platform (ICTRP) taps into a global network of knowledge.
The European Union has undergone a major regulatory shift. The EU Clinical Trials Register (EUCTR) contains information on over 34,000 interventional trials conducted in the EU under the old directive. However, as of 2022, the new EU Clinical Trial Regulation (CTR) requires all new trials to be registered in the Clinical Trials Information System (CTIS). This new, unified portal streamlines the application and supervision process and aims to increase transparency by making more trial information public by default. You can search the old register here: EU Clinical Trials Register, while CTIS represents the future of European trial transparency.
The ISRCTN registry, with over 18,000 studies, is a UK-based global registry recognized by the WHO. It is notable for providing plain English summaries alongside scientific protocols, making information accessible to a non-medical audience. Explore it at the ISRCTN registry.
Other key national and regional registries include:
- The Pan African Clinical Trial Registry (PACTR), a hub for trials in Africa, providing global visibility for African research. See the registry here: Pan African Clinical Trial Registry (PACTR).
- The Chinese Clinical Trial Registry (ChiCTR), a primary registry in the WHO network, is essential for accessing data on the vast number of trials conducted in China.
- The Japan Registry of Clinical Trials (jRCT) serves a similar function for trials conducted in Japan.
- The Australian New Zealand Clinical Trials Registry (ANZCTR) is the main repository for trials in that region.
Together, these registries form an interconnected web of clinical trial data that accelerates access to life-saving treatments and fosters global scientific collaboration.
The Push for Transparency: Benefits, Challenges, and Key Players

Transparency in clinical research is a crucial, hard-won development in modern medicine. Openly accessible clinical trial data transforms how we understand and develop treatments. For decades, publication bias—the tendency for studies with positive or statistically significant results to be published over those with negative or inconclusive findings—created a dangerously misleading picture of treatment effectiveness and safety.
Modern transparency initiatives combat this by ensuring a more complete evidence base. Access to all clinical trial data, including negative or neutral results, allows for data re-analysis to validate findings, explore subgroups, and generate new hypotheses. It is the foundation for systematic reviews and meta-analyses, like those conducted by Cochrane, which synthesize all available evidence to provide the most reliable assessment of a treatment. However, the path to full transparency faces significant challenges. A primary hurdle is the lack of standardization. Data from different trials is often collected and formatted differently, making it complex to aggregate and compare. The data quality can also be highly variable. A further challenge is balancing transparency with the need to protect commercially confidential information (CCI), a major concern for pharmaceutical sponsors who invest heavily in research and development.
The Role of NIH and CTTI
Two key organizations lead the charge for better clinical trial data.
The National Institutes of Health (NIH) is a major driver of data sharing. As the largest public funder of biomedical research in the world, its policies have a profound impact. Besides funding studies, the NIH maintains a database of its research, which you can explore at Clinical Trials and Research Studies at the NIH. The NIH Policy for Data Management and Sharing now requires all funded research to include a robust plan for managing and sharing data.
The Clinical Trials Transformation Initiative (CTTI), a public-private partnership co-founded by Duke University and the FDA, focuses on improving data quality and trial efficiency. CTTI brings together stakeholders from across the research enterprise to develop and drive adoption of practices that will increase the quality and efficiency of clinical trials. Their work on data sharing provides practical guidance, with resources available in their CTTI publications on data sharing.
Ethical Considerations and Regulatory Requirements
Transparency in clinical trial data must balance openness with the paramount need for patient protection, all governed by a framework of ethical and regulatory guidelines.
Informed consent is the ethical foundation. It is a process, not just a form, ensuring participants understand the trial’s purpose, risks, benefits, and how their data will be collected, stored, and potentially shared for future research. Data sharing policies like FDAAA 801 in the US and the EU’s Clinical Trial Regulation mandate public disclosure of most trial results, regardless of outcome, to combat publication bias and ensure regulatory compliance. Ethical oversight from bodies like Institutional Review Boards (IRBs) in the US and Research Ethics Committees (RECs) elsewhere is crucial. These independent committees review trial protocols, consent forms, and recruitment materials before a study begins and continue to monitor its conduct to protect the rights, safety, and well-being of participants. For more insights, explore more info about celebrating data in clinical trials.
Protecting Patients and Powering Progress

Clinical trial data holds the key to medical breakthroughs, but we must open up its insights without compromising patient privacy. When volunteers share their health information, we have a responsibility to honor that trust while enabling scientific innovation.
How Patient Privacy is Protected
Protecting patient privacy in clinical trial data relies on data anonymization and de-identification. Anonymization removes all identifiable information, while de-identification removes direct identifiers but may retain indirect ones for research. Protection begins with the informed consent process, where participants learn how their data will be used. Data is then stored in secure data environments with strict access controls and governance models.
At Lifebit, our federated AI system embodies this principle, allowing real-time access to global biomedical data while upholding the highest privacy standards. Learn more in our guides on secure data platforms and clinical trial patient data protection.
How Pharmaceutical Companies Approach Data Sharing
Pharmaceutical companies are increasingly leading clinical trial data transparency efforts, driven by regulations, ethics, and the realization that sharing accelerates progress.
Pfizer is a leader in this area, committed to sharing all trial results, regardless of outcome. See their approach at Pfizer’s data transparency portal. A prime example of industry collaboration is ClinicalStudyDataRequest.com, where companies like Astellas, Bayer, and GSK pool their data for researchers to request. Companies like Biogen and Sanofi also make detailed trial results available. This shift toward sharing positive and negative results is a fundamental change that accelerates medical progress for everyone.
Advanced Tools and Common Pitfalls for Researchers
The world of clinical trial data has opened up, but navigating this vast ocean requires the right tools and a keen awareness of common pitfalls. Raw access isn’t enough; researchers need sophisticated tools to analyze millions of data points and steer clear of traps that can lead to flawed conclusions.
Enhancing Access with AACT
The AACT (Aggregate Analysis of ClinicalTrials.gov) database is a game-changer for researchers. It’s a publicly available relational database that contains all information from ClinicalTrials.gov, parsed into a structured format and refreshed daily. AACT is special for its openness and power, providing open-source tools and source code on Github. This allows researchers to bypass the web interface and use technologies like PostgreSQL for sophisticated data analysis. For example, a researcher could write a SQL query to identify all Phase 3 oncology trials completed in the last five years, join this with results data to extract primary endpoint details, and analyze the prevalence of specific biomarkers used in eligibility criteria. AACT is accessible to all researchers, and its daily refresh ensures the data is always current. For more on how technology is changing research, read about clinical research SaaS technology.
Common Problems When Using Clinical Trial Data
Despite advances, researchers face common problems that can undermine the validity of their analyses of clinical trial data.
- Data interpretation errors: Studies may use similar terms for different meanings (e.g., “progression-free survival” might be defined differently across trials). Endpoint switching, where the primary endpoint is changed during the trial, can also be a major source of bias if not transparently reported and accounted for.
- Inconsistent data: Reporting standards and definitions can vary between trials and over time. Efforts like the Clinical Data Interchange Standards Consortium (CDISC) aim to solve this by creating universal standards (e.g., SDTM for submission data, ADaM for analysis data), but their adoption is not yet universal.
- Missing data: Gaps from participant dropouts or uncollected data points are nearly universal and can seriously bias results if not handled correctly. It’s crucial to understand the type of missingness: Missing Completely at Random (MCAR), Missing at Random (MAR), or Missing Not at Random (MNAR). Sophisticated statistical techniques like multiple imputation are often required to address this.
- Protocol amendments: Trials are not static; their protocols are often amended due to slow recruitment, new safety information, or other logistical reasons. An analyst unaware of a major amendment—like a change in dose or eligibility criteria—can draw incorrect conclusions by pooling data from before and after the change.
- Data quality issues: Even with rigorous checks, errors like typos, impossible values (e.g., a patient’s height listed as 10 feet), or inconsistent units (e.g., weight in pounds vs. kilograms) can occur and skew analyses.
- Selection Bias: The population enrolled in a clinical trial is often not representative of the broader patient population due to strict eligibility criteria. This can limit the generalizability of the trial’s findings to real-world clinical practice.
The Clinical Trials Transformation Initiative (CTTI) offers guidance on how to avoid common problems when using ClinicalTrials.gov. Success requires approaching clinical trial data with both enthusiasm and a healthy dose of skepticism. Understand the data’s provenance, validate interpretations, and always remember that each data point represents a person who contributed to advancing medicine.
Frequently Asked Questions about Clinical Trial Data
How can I find a clinical trial to participate in?
Several excellent resources can help you find the right clinical trial. Your first and best step should always be a conversation with your doctor or healthcare team.
- Your doctor is a trusted partner who knows your medical history, can help determine if a trial is a suitable option for you, and can explain the specific eligibility criteria in the context of your health.
- ClinicalTrials.gov is the most comprehensive starting point for an independent search. You can search by condition, location, drug name, or other keywords. For cancer-related trials, the National Cancer Institute has a specialized search for NIH clinical trials for cancer.
- ResearchMatch is a free, secure registry funded by the NIH that connects volunteers with researchers who are looking for participants for their studies.
- Disease-specific organizations (e.g., the American Heart Association, the Michael J. Fox Foundation for Parkinson’s Research) often maintain curated lists of ongoing trials relevant to their communities.
Always discuss any potential study you find with your healthcare team to ensure it is a safe and appropriate choice for you.
Are all clinical trial results made public?
Transparency of clinical trial data has improved significantly, but the answer is still, “not always, but much more often than before.” Regulatory requirements, like FDAAA 801 in the U.S. and the new CTR in the EU, mandate that most trial results be reported to public databases like ClinicalTrials.gov within a specific timeframe (usually one year after completion), regardless of the outcome. This helps combat publication bias. Many pharmaceutical companies and research institutions also have policies for voluntarily sharing all trial results. However, gaps remain. Older trials conducted before these mandates may not have public results. Delays in reporting can occur, and some types of studies (e.g., early-phase trials) may be exempt from mandatory reporting requirements. The bottom line is that results from most recent, later-phase trials are very likely to be found in public registries, a major step forward for science.
What is the difference between clinical trial data and real-world data?
Clinical trial data (CTD) and real-world data (RWD) are two distinct but increasingly complementary sources of evidence about medical treatments.
Clinical trial data is generated within the controlled environment of a clinical trial, which is a type of interventional study. Its primary purpose is to determine the efficacy and safety of a new intervention under ideal conditions.
- Data Source: Collected specifically for the trial using Case Report Forms (CRFs).
- Study Design: Interventional, with a pre-defined protocol, strict eligibility criteria, and often randomization and blinding.
- Population: A highly selected, homogeneous group of participants.
- Data Collection: Rigorous, standardized, and complete for the specified endpoints.
- Strength: High internal validity; excellent for establishing cause-and-effect relationships.
- Limitation: Limited generalizability to the broader, more diverse patient population seen in routine care.
Real-world data is collected from sources outside of traditional clinical trials, reflecting what actually happens in everyday medical practice. It is typically generated through observational studies.
- Data Source: Electronic health records (EHRs), medical claims and billing data, product and disease registries, data from wearables and mobile devices, and patient-reported surveys.
- Study Design: Observational; researchers analyze data from routine clinical practice without assigning interventions.
- Population: A large, heterogeneous, and diverse population representative of real-world patients.
- Data Collection: Can be messy, unstructured, and incomplete.
- Strength: High external validity or generalizability; excellent for understanding long-term safety, treatment patterns, and effectiveness in diverse populations.
- Limitation: Lower internal validity; susceptible to bias and confounding, making it difficult to establish causality.
Both data types are crucial. Clinical trials provide the high-quality evidence needed for regulatory approval. RWD, and the real-world evidence (RWE) derived from it, shows how the treatment performs in the broader population over the long term. Increasingly, these two worlds are merging. RWD is used to design more efficient trials, for example, by creating synthetic or external control arms to compare against the investigational arm, reducing the need for placebo groups. For a deeper dive, check out our info on Real-World Data in clinical trials.
Conclusion
We’ve journeyed through clinical trial data, from decoding trial records to mapping global registries, uncovering how this information powers medical progress. We’ve seen how organizations like the NIH and CTTI champion transparency, how patient privacy is protected, and how pharmaceutical companies are opening their data vaults. By sharing all findings, they create a more complete picture of medical research.
This transparency changes medical innovation. It allows researchers to build on existing knowledge, enables informed decisions, and prevents publication bias, creating a foundation for faster, smarter breakthroughs.
The future is even more exciting, with federated data access and advanced AI analytics breaking down data silos. This will enable real-time insights across global datasets while maintaining privacy and compliance.
This vision drives us at Lifebit. Our federated AI platform, with its Trusted Research Environments (TREs) and Real-time Evidence & Analytics Layer (R.E.A.L.), powers large-scale, compliant research. We harmonize datasets, enable secure collaboration, and accelerate findy. The goal is a future where medical progress has no boundaries, insights flow freely but securely, and breakthroughs come from connecting previously unlinked data.
Ready to open up the full potential of clinical trial data and transform your research capabilities? Accelerate your research with advanced data solutions and see how our platform can help you turn data into findies.

