Clinical Trial Recruitment Strategies to Fill Your Study Faster

Clinical Trial Recruitment Strategies: Boost Enrollment 2025
Why Clinical Trial Recruitment Success Determines Healthcare Innovation
Clinical trial recruitment strategies are the backbone of medical breakthroughs, yet most trials struggle to find enough participants. The stakes are high: two-thirds of trials fail to meet enrollment targets, costing the industry $40 billion annually and delaying life-saving treatments by 10-15 years.
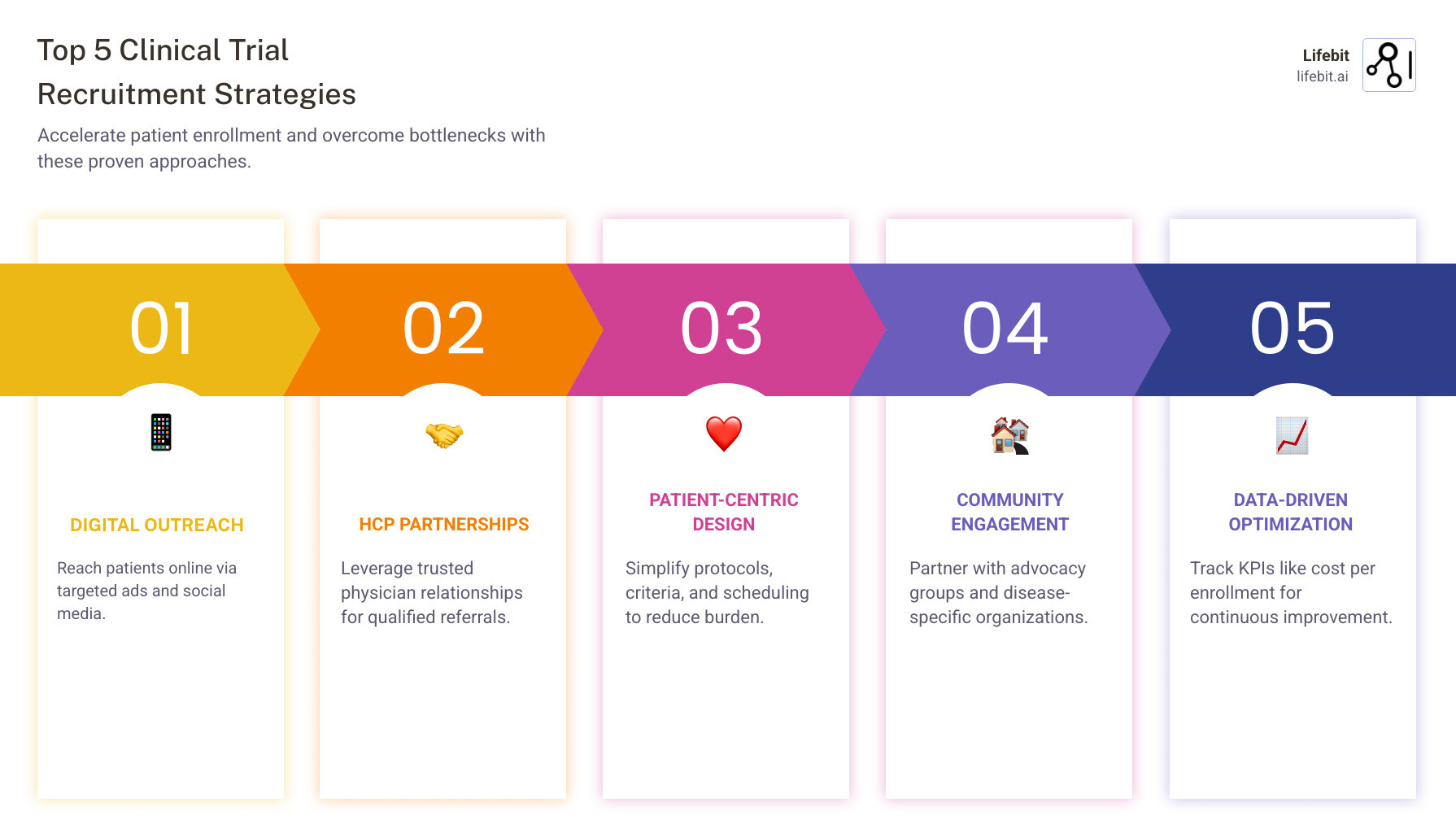
Traditional methods are too slow. Success now requires a multi-faceted approach:
- Digital-first outreach to reach patients online.
- Healthcare provider partnerships for trusted referrals.
- Patient-centric protocol design to reduce participant burden.
- Community engagement with advocacy groups.
- Data-driven optimization to track key metrics.
I’m Maria Chatzou Dunford, CEO of Lifebit. My 15+ years in the field have shown me that effective recruitment hinges on connecting the right data with the right patients at the right time.
Essential clinical trial recruitment strategies terms:
- digital clinical trial recruitment
- clinical trial recruitment digital case study
- clinical trial recruitment digital results
The High Stakes of Recruitment: Why Traditional Methods Fall Short
Effective clinical trial recruitment strategies can make or break medical breakthroughs. Unfortunately, two-thirds of clinical trials fail to meet their enrollment targets, creating a systemic crisis. This failure results in a staggering $40 billion annual loss for the industry and, more importantly, forces patients to wait an additional 10-15 years for new treatments.
Scientific research on trial accrual failures confirms that these are predictable outcomes of relying on outdated methods. Traditional approaches like newspaper ads and hospital flyers cannot keep pace with the demands of precision medicine, which requires finding patients with specific characteristics.
Common Recruitment Barriers
Understanding these common obstacles is the first step toward building better clinical trial recruitment strategies:
- Strict eligibility criteria: Unnecessarily narrow requirements can severely limit the pool of potential participants.
- Lack of patient awareness: Many people are unaware that clinical trials are a treatment option or how to find them.
- Geographic limitations: Requiring frequent travel to research sites is a major hurdle for many patients.
- Patient mistrust: Historical ethical violations have created lasting skepticism, particularly in minority communities.
- Logistical burdens: Time away from work, complex procedures, and multiple appointments can overwhelm participants.
- Competition between trials: Multiple studies often compete for the same small group of eligible patients.
The solution requires a shift in perspective: from “finding patients for our trial” to “designing trials that work for our patients.”
Foundational Strategies: Patient-Centricity and Protocol Design
Successful clinical trial recruitment strategies treat patients as partners, not subjects. This patient-centric approach focuses on designing trials that are manageable for participants, which reduces burden and improves both enrollment and retention. It involves mapping the entire patient journey to identify and address potential obstacles.
Designing Patient-Friendly Protocols
A patient-friendly protocol is essential for recruitment. Key elements include:
- Simplified inclusion/exclusion criteria: Every criterion should be necessary. Loosening just one restriction can significantly expand the patient pool.
- Realistic visit schedules: Consolidate procedures and reduce the frequency of check-ins to respect patients’ time.
- Incorporating patient feedback: Engage patient advocacy groups during protocol development to identify potential burdens.
- Decentralized trial elements: Use home healthcare, local labs, and direct-to-patient shipments to reduce travel.
- Remote monitoring: Employ wearables and telehealth for patient convenience and richer data collection.
Integrating Retention into Your Clinical Trial Recruitment Strategies
Recruitment and retention planning must be blended. A recruited patient who drops out is a loss for the study.
- Building trust from day one: Use the informed consent process to establish a partnership.
- Clear communication: Keep patients informed about study progress and next steps.
- Setting realistic expectations: Be transparent about time commitments and potential side effects.
- Support services: Offer practical help like transportation stipends or flexible scheduling.
- Reminder systems: Use automated reminders for appointments and medication to help patients stay on track.
- Integrating retention KPIs: Track metrics like withdrawal rates and patient satisfaction to identify issues early.
Modernizing Outreach: Digital Clinical Trial Recruitment Strategies
Your clinical trial recruitment strategies must be digital because that’s where patients are. People research health conditions and treatments online, making digital channels the most effective way to connect. Digital methods are highly cost-effective, with some costing as little as $92 per enrollment compared to thousands for traditional approaches.
| Feature | Traditional Recruitment Methods | Digital Recruitment Methods |
|---|---|---|
| Cost | $500-$5,000+ per enrollment | $92-$500 per enrollment |
| Reach | Limited to local geographic areas | Global reach with precise targeting |
| Speed | Weeks to months for results | Real-time engagement and faster enrollment |
| Targeting | Broad, demographic-based | Precise targeting by condition, interests, behavior |
| Tracking | Difficult to measure effectiveness | Detailed analytics and performance metrics |
| Flexibility | Hard to modify once launched | Easy to adjust campaigns in real-time |
This is about meeting patients where they are, at the moment they are seeking information and hope.
Leveraging Digital Advertising and Social Media
Targeted digital advertising delivers trial information directly to relevant audiences.
- Google and Meta Ads: Use platforms like Google and Facebook to reach people based on search history, interests, and demographics. Use patient-friendly language, not medical jargon.
- IRB Approval: All ad copy and creative must be approved by an Institutional Review Board (IRB) before launch.
- A/B Testing and Analytics: Continuously test different messages and use tools like Google Analytics to track performance. This allows you to optimize your budget by focusing on what works.
Utilizing Patient Registries and Online Communities
Tap into existing pools of motivated individuals.
- Patient Registries: Use resources like ResearchMatch, a free national registry connecting volunteers with researchers.
- ClinicalTrials.gov: This is a primary tool for patients. Ensure your listing is complete, easy to understand, and regularly updated with responsive contact information.
- Online Communities: Disease-specific forums and support groups are valuable but require a respectful approach. Build trust by providing helpful information before promoting your trial.
Platforms offering Real-World Evidence capabilities, like Lifebit, can further improve participant identification by securely analyzing diverse data sources.
Introduction

Every medical breakthrough begins with a clinical trial, but the greatest hurdle is often not the science—it’s finding enough participants. This recruitment bottleneck significantly slows medical progress.
The statistics are stark: two-thirds of clinical trials fail to meet enrollment targets. This leads to delays that cost the industry $40 billion annually and leave patients waiting 10-15 years longer for life-saving treatments.
Traditional methods like print ads and flyers are no longer sufficient. Success now hinges on modern clinical trial recruitment strategies that are both smarter and more efficient. Digital recruitment, for example, can cost as little as $92 per enrollment, a fraction of traditional costs.
I’m Maria Chatzou Dunford, CEO of Lifebit. My experience has shown that overcoming this challenge requires connecting the right data with the right patients at the right time. This guide explores the strategies that blend technology with a deep understanding of the patient journey to accelerate medical innovation.
Essential clinical trial recruitment strategies resources:
- digital clinical trial recruitment
- clinical trial recruitment digital case study
- clinical trial recruitment digital results
The High Stakes of Recruitment: Why Traditional Methods Fall Short
Patient recruitment challenges are not minor problems; they are monumental problems that delay life-saving innovations. The fact that two-thirds of clinical trials fail to meet enrollment targets creates a systemic bottleneck with severe consequences. The financial impact is an estimated $40 billion annual loss for the industry. This staggering figure stems from extended site maintenance fees, prolonged staff salaries, the cost of failed recruitment campaigns, and, most significantly, the lost revenue from a delayed market entry. For a blockbuster drug, a single day’s delay can represent millions in lost sales.
However, the human cost is far greater. A 10-15 year longer wait for new therapies is a devastating statistic for patients with progressive or terminal illnesses. For them, a clinical trial can represent the last and best hope. Delays mean that hope is deferred or denied entirely. Scientific research on trial accrual failures confirms these are systemic issues, not isolated incidents. Traditional methods like print, radio, and general flyers are increasingly ineffective. They lack the precision needed for modern research, which often requires finding patients with specific genetic markers or complex disease histories.
Common Recruitment Barriers
Overcoming these challenges requires a deep understanding of the barriers that prevent patients from participating. Effective clinical trial recruitment strategies are built on dismantling these obstacles one by one.
- Strict Eligibility Criteria: While necessary for scientific validity, overly restrictive inclusion/exclusion criteria are a primary cause of recruitment failure. A trial for a specific lung cancer mutation might exclude patients with well-managed diabetes or those over a certain age, drastically shrinking the potential participant pool without a clear scientific justification.
- Lack of Patient Awareness: Many people, and even some physicians, are unaware that clinical trials are a viable care option. A survey by the Center for Information and Study on Clinical Research Participation (CISCRP) found that while 80% of patients were willing to participate in research, fewer than half felt they were well-informed about how to find and join a trial.
- Geographic Limitations: The traditional model requires patients to travel to a specific research site, often in a major city. This is a significant burden, involving costs for travel, lodging, and parking, as well as time away from work and family. For patients in rural areas or those with mobility issues, this barrier can be impossible.
- Patient Mistrust: A painful history of ethical violations in medical research, such as the Tuskegee Syphilis Study, has created a deep and lasting skepticism, particularly within minority and underserved communities. Rebuilding this trust is a long-term commitment that requires transparency, community engagement, and demonstrable respect for participants.
- Logistical Burdens: Participation can be overwhelming. The demands of multiple appointments, long wait times at the clinic, complex procedures, and arranging for childcare or elder care can lead to high dropout rates even among enrolled patients.
- Competition Between Trials: In many therapeutic areas, especially oncology and rare diseases, multiple trials are often competing for the same small, highly specific group of eligible patients. This creates a zero-sum game where one trial’s success can mean another’s failure.
Addressing these barriers requires a fundamental paradigm shift: from a trial-centric mindset of “finding subjects for our study” to a patient-centric one focused on “designing studies that work for patients.”
Foundational Strategies: Patient-Centricity and Protocol Design
At the heart of all successful clinical trial recruitment strategies is the principle of patient-centricity. This is more than a buzzword; it is a fundamental commitment to designing and conducting research with the patient’s experience, needs, and perspective as the primary focus. It means treating participants as active partners in the research process, not as passive subjects. By reducing participant burden and incorporating patient insights from the very beginning, sponsors can dramatically improve both enrollment rates and long-term retention.
Designing Patient-Friendly Protocols
The protocol is the blueprint for the trial, and a poorly designed one can doom recruitment from the start. To optimize recruitment, protocols must be as patient-friendly as scientifically possible.
- Simplified Inclusion/Exclusion Criteria: Every single criterion should be critically evaluated. Ask: “Is this requirement absolutely essential to answer the primary research question?” Often, criteria related to comorbidities or concomitant medications can be loosened without compromising data integrity, significantly expanding the eligible patient pool.
- Realistic Visit Schedules: Minimize the disruption to patients’ lives. This can be achieved by consolidating procedures into fewer visits, offering flexible scheduling windows (including evenings or weekends), and clearly communicating the time commitment for each visit.
- Incorporating Patient Feedback: Engage patients and patient advocacy groups (PAGs) during the protocol development phase, not after it’s finalized. Use patient advisory boards, focus groups, and surveys to identify potential burdens and gather insights on what would make participation more manageable.
- Remote Monitoring: Leverage technology to collect data conveniently and continuously. Wearable devices (like smartwatches and fitness trackers) and other sensors can capture real-world data on activity levels, sleep patterns, and vital signs, often providing richer insights than periodic clinic visits.
Embracing Decentralized Clinical Trials (DCTs)
One of the most powerful ways to make a trial patient-centric is to incorporate decentralized elements. DCTs use technology to bring the trial to the patient, rather than forcing the patient to come to the trial.
- Models of Decentralization: DCTs exist on a spectrum. A hybrid DCT is the most common model, combining traditional site visits for complex procedures with decentralized components like home health visits, local lab testing, and telehealth check-ins. A fully decentralized or virtual trial is conducted entirely remotely, with no physical site visits required.
- Key Technologies: DCTs are enabled by a suite of digital tools, including eConsent (electronic informed consent), ePRO (electronic patient-reported outcomes collected via apps or web portals), telehealth platforms for virtual visits, and direct-to-patient (DtP) logistics for shipping study drugs and supplies.
- Benefits: The advantages are significant. DCTs reduce geographic barriers, which can dramatically increase the size and diversity of the potential participant pool. They also improve patient convenience, which can lead to higher retention rates.
Integrating Retention into Your Clinical Trial Recruitment Strategies
Recruitment is only half the battle. Effective clinical trial recruitment strategies must include a robust retention plan from day one, as a patient who drops out represents a significant loss of data, time, and resources.
- Building Trust from Day One: The informed consent process should be a conversation, not a transaction. Ensure patients fully understand the study and have ample opportunity to ask questions. This builds a foundation of trust.
- Clear and Consistent Communication: Keep patients informed and engaged. Provide regular updates on the study’s progress (in aggregate, non-blinding terms), and use a dedicated patient navigator or coordinator as a single point of contact.
- Setting Realistic Expectations: Be transparent about the time commitments, potential side effects, and procedures involved. Surprises lead to frustration and dropouts.
- Comprehensive Support Services: Think beyond the science. Offer practical support like transportation stipends, meal vouchers, or reimbursement for childcare costs. These small gestures show that you value the patient’s contribution.
- Automated Reminder Systems: Use text, email, or app-based reminders to help patients stay on track with appointments, medication schedules, and diary entries. This improves compliance and reduces the mental load on participants.
- Integrating Retention KPIs: Actively monitor metrics like withdrawal rates and patient satisfaction surveys. If you see a problem emerging at a particular site or phase of the trial, you can intervene early to address it.
Modernizing Outreach: Digital Clinical Trial Recruitment Strategies
The world has gone digital, and so must our clinical trial recruitment strategies. Today’s patients are e-patients; they are empowered, informed, and actively use the internet to research their health conditions, explore treatment options, and connect with others who share their diagnosis. Digital platforms offer unparalleled precision, reach, and cost-effectiveness. While traditional methods can cost thousands per enrolled patient, well-executed internet recruitment campaigns can cost as little as $92 per enrollment, and social media campaigns can achieve a 92.3% completion rate for engagement activities like pre-screeners.
| Feature | Traditional Recruitment Methods | Digital Recruitment Methods |
|---|---|---|
| Cost | High upfront investment (print, TV ads) with uncertain ROI | Lower cost-per-acquisition, highly measurable ROI |
| Reach | Limited to local media circulation or broadcast area | Global, highly scalable, and not bound by geography |
| Speed | Slow lead times for creative development and placement | Fast, real-time deployment and automated outreach |
| Targeting | Broad and imprecise (e.g., readers of a specific magazine) | Hyper-precise (demographics, location, interests, online behaviors, search history) |
| Measurability | Difficult to track which ad led to an enrollment | Real-time analytics, clear attribution from click to enrollment |
| Engagement | One-way, passive communication (e.g., a flyer) | Interactive, two-way communication and community building |
This digital shift also enables the use of real-world data to identify participants more efficiently. More info about Real-World Evidence solutions can transform recruitment from educated guesswork into data-driven precision targeting.
Leveraging Digital Advertising and Social Media
Use digital advertising to cast a smarter, more effective net. Platforms like Google and Meta allow you to target users with incredible granularity, reaching them at the exact moment they are seeking information and hope.
- Platform-Specific Tactics: On Google Ads, use search campaigns to target users actively looking for terms like “new treatments for Crohn’s disease” or “lung cancer clinical trials.” Use display campaigns to place ads on relevant health websites and forums. On Meta (Facebook/Instagram), build audiences based on interests (e.g., users who follow specific advocacy groups), demographics, and lookalike audiences modeled on your existing patient profiles.
- Patient-Centric Content: All advertising must use patient-friendly language. Avoid complex medical jargon. Focus on the potential benefits and what participation entails in simple, clear terms. Use compelling visuals and consider video testimonials from past participants or the principal investigator.
- IRB Approval and Optimization: All ad copy, images, and landing pages must be reviewed and approved by an Institutional Review Board (IRB) to ensure they are ethical and not coercive. Once live, use A/B testing to continuously optimize. Test different headlines, images, and calls-to-action to see what resonates most with your target audience. Use analytics tools to monitor performance and reallocate your budget to the best-performing channels and ads.
Utilizing Patient Registries and Online Communities
Engage with existing patient ecosystems where motivated individuals are already gathered.
- Patient Registries: Leverage databases like ResearchMatch, which connects researchers with a large pool of willing volunteers across the U.S. Many disease-specific foundations also maintain their own patient registries.
- ClinicalTrials.gov: This is often the first stop for patients seeking trials. Your listing must be a priority. Write a clear, plain-language summary. Ensure the contact information is for a responsive person or team, not a generic inbox. Keep the recruitment status updated diligently.
- Online Communities and Support Groups: Platforms like PatientsLikeMe, disease-specific subreddits, and Facebook groups are invaluable. However, engagement must be respectful and authentic. Do not simply post a link to your trial. Become part of the community, provide helpful information, and build trust. Always check the group’s rules and engage with moderators before posting about a research opportunity.
The Role of AI and Machine Learning
Artificial intelligence is emerging as a powerful tool in modern recruitment. AI algorithms can analyze vast datasets—such as de-identified Electronic Health Records (EHRs) and real-world data—to identify potential participants who meet complex eligibility criteria. This process, known as AI-powered patient-to-trial matching, can drastically reduce the time and manual effort required for site-level chart review. Furthermore, machine learning models can predict enrollment rates at different sites, helping sponsors allocate resources more effectively and identify potential recruitment bottlenecks before they occur.
Building a Recruitment Ecosystem: Leveraging Partnerships and Trust
No trial recruits in a vacuum. The most effective clinical trial recruitment strategies are built on an ecosystem of collaboration, community, and trust. This involves moving beyond transactional relationships to build genuine partnerships with healthcare providers, patient advocates, and the communities you aim to serve. Success hinges on engaging these stakeholders early, communicating transparently, and demonstrating an unwavering commitment to ethical conduct and diversity.
Collaborating with Healthcare Providers (HCPs)
HCPs are the most trusted source of health information for patients. A recommendation from a personal physician is one of the most powerful drivers of trial participation. A CISCRP survey confirmed this, showing that 64% of patients prefer to hear about clinical trials from their doctor.
- Overcoming Referral Barriers: To earn referrals, you must first understand why HCPs might be hesitant. Common barriers include a lack of time to stay informed about available trials, fear of losing the patient from their practice, and uncertainty about the trial’s protocol or potential impact on the patient’s care.
- Empowering Physicians with Information: Build strong relationships with local specialists and primary care physicians. Equip them with a “physician outreach toolkit” that includes a one-page trial summary, a clear protocol synopsis, inclusion/exclusion criteria at a glance, and a simple, direct referral pathway. This makes it easy for a busy clinician to identify a suitable candidate and make a confident referral.
Partnering with Patient Advocacy Groups (PAGs)
PAGs are essential allies. They are deeply connected to motivated and informed patient communities and can serve as a bridge of trust between researchers and participants.
- Access to Motivated Patients: PAGs are natural hubs for individuals who are actively seeking new treatments and are eager to contribute to research.
- Building Credibility and Trust: A partnership with a respected PAG lends immediate credibility to your study. Their endorsement acts as a seal of approval, assuring patients that the trial is legitimate and valuable.
- Co-creating Recruitment Materials: Work directly with PAGs to develop recruitment materials. Their insights are invaluable for ensuring that language is culturally sensitive, respectful, and easy to understand. They can then help disseminate these co-created materials through their trusted channels, such as newsletters, websites, social media, and patient conferences.
A Proactive Approach to Diversity and Inclusion
Ensuring diversity in clinical trials is not just an ethical imperative; it is a scientific necessity. A treatment that is safe and effective in one population may not be in another. Historically, many groups—including racial and ethnic minorities, women, the elderly, and rural populations—have been underrepresented in research. A modern recruitment strategy must actively work to correct this.
- Strategic Site Selection: Place trial sites in communities with diverse populations, not just at major academic centers.
- Community-Based Participatory Research (CBPR): Engage community leaders, faith-based organizations, and local clinics from the start. Build relationships based on mutual respect and listen to the community’s concerns and needs.
- Culturally Competent Communication: Translate all study materials into relevant languages. Ensure that imagery and messaging are inclusive and resonate with the communities you are trying to reach.
- Address Systemic Barriers: Offer flexible scheduling, provide transportation or reimbursement, and use decentralized trial elements to reduce the burden on participants who may have less flexible jobs or limited access to transportation.
Upholding the Highest Ethical Standards in Recruitment
Trust is the currency of clinical research. It is hard-won and easily lost. Adhering to strict ethical standards is non-negotiable for all clinical trial recruitment strategies.
- Informed Consent as a Process: The informed consent process must be clear, comprehensive, and entirely free from pressure. It is an ongoing dialogue that ensures a participant understands the risks, benefits, and alternatives, allowing them to make a fully informed decision. The use of eConsent with multimedia elements can aid comprehension.
- Rigorous IRB Oversight: Every single component of your recruitment plan—from social media ads and flyers to consent forms and compensation plans—must be reviewed and approved by an Institutional Review Board (IRB) before use.
- Avoiding Undue Influence: Compensation should reimburse participants for their time and travel, but it must not be so high as to coerce or unfairly persuade individuals to take on risks they would otherwise refuse.
- Commitment to Transparency and Privacy: Be honest and transparent in all communications. Strictly adhere to data privacy regulations like GDPR and HIPAA to protect sensitive patient information. For more guidance, refer to resources like the HRPP Policy on Recruiting Methods.
Measuring and Optimizing Your Success
Successful clinical trial recruitment strategies are not static; they are dynamic, data-driven, and continuously optimized. You cannot improve what you do not measure. By systematically tracking key metrics across the entire recruitment funnel, you can identify what’s working, diagnose problems early, and make informed decisions to allocate resources effectively. This iterative process of measuring, analyzing, and refining is what separates high-performing trials from those that fall behind.
Key Performance Indicators (KPIs) for Recruitment
Tracking the right KPIs provides a clear, objective view of your recruitment performance. A real-time dashboard displaying these metrics is an essential tool for any study manager.
- Funnel Conversion Rates: Track the percentage of patients who advance from one stage to the next (e.g., website visitors to pre-screener completion, pre-screened to consented, consented to enrolled). This helps pinpoint exactly where in the process you are losing potential participants.
- Cost per Screened/Enrolled Patient: This measures the financial efficiency of your campaigns. Tracking this by channel (e.g., cost per enrollment from Google Ads vs. physician referrals) reveals your most cost-effective sources.
- Screen Failure Rate: A high screen failure rate (the percentage of consented patients who are found to be ineligible) is an early warning sign that your targeting is off or your eligibility criteria are too restrictive.
- Enrollment Rate per Site: This helps identify high-performing sites that could be models for others, as well as underperforming sites that may need additional support, training, or resources.
- Time to First/Last Patient In: These metrics measure the overall velocity of your recruitment and are critical for timeline management.
- Source of Enrollment Tracking: Carefully track where each enrolled patient came from. This data is crucial for calculating the ROI of each channel and optimizing future budget allocation.
- Diversity Metrics: Track enrollment by race, ethnicity, gender, and age against your pre-defined diversity goals. This ensures you are accountable for building a representative cohort.
Best Practices for Engaging Potential Participants
How your team engages with potential participants is a critical factor in conversion and retention. Every interaction is an opportunity to build trust and demonstrate respect.
- Use Clear and Simple Language: Medical jargon is intimidating and can create a power imbalance. All communication, from ads to consent forms, should be written at an 8th-grade reading level or lower. Use tools to assess health literacy and ensure your message is accessible to everyone.
- Offer Multi-Channel Communication: Meet people where they are. Allow potential participants to connect via their preferred method, whether it’s a phone call, email, web chat, or text message. Ensure a seamless experience across all channels.
- Maintain a Responsive and Empathetic Pre-Screening Team: The first human contact is often with a pre-screening specialist or call center. This team must be well-trained, empathetic, and responsive. A prompt, professional, and compassionate response can convert initial interest into genuine engagement. Aim to respond to all inquiries within 24 hours.
- Provide a Graceful Exit for Non-Eligible Participants: Most people who inquire about a trial will not be eligible. Do not treat this as a dead end. Thank them sincerely for their interest, explain politely why they are not a fit for this particular study, and ask for their permission to be added to a registry for future research opportunities. This builds immense goodwill and creates a valuable asset for future trials.
- Send Regular Updates to Enrolled Patients: Communication should not stop after enrollment. Keep participants informed and feeling valued throughout the trial. Regular newsletters with aggregate study updates (that don’t break the blind) can significantly improve retention and make participants feel like true partners in the research.
Frequently Asked Questions about Clinical Trial Recruitment
Here are detailed answers to some of the most common questions about developing and executing effective clinical trial recruitment strategies.
What is the most cost-effective way to recruit for a clinical trial?
The most cost-effective method is highly dependent on the specific trial, therapeutic area, and patient population. However, the highest ROI is typically achieved through a multi-channel strategy that blends different approaches. Physician referrals often have the lowest direct cost per enrollment (sometimes as low as $12) and yield highly qualified candidates, but they are not easily scalable. Digital approaches like targeted social media or search engine advertising (which can be as low as $92 per enrollment) offer incredible scalability and reach but may have lower conversion rates. The optimal strategy is to use broad digital outreach to generate awareness and initial interest, then nurture those leads through high-touch channels like a dedicated call center and partnerships with referring physicians. The key is to track the cost-per-enrollment for each channel continuously and reallocate the budget toward the channels that provide the best return on investment for your specific study.
How can I improve diversity in my clinical trial?
Improving diversity is a critical ethical and scientific goal. A passive approach is not enough; you need proactive clinical trial recruitment strategies focused on inclusion. Key actions include:
- Engage Communities Directly: Don’t wait for patients to come to you. Partner with community leaders, faith-based organizations, and local clinics in underserved areas. Build trust over time by listening to their needs and participating in community health events.
- Strategic Site Selection: Intentionally select research sites in geographic areas with diverse populations.
- Culturally Competent Materials: Translate all patient-facing materials (ads, brochures, consent forms) into the primary languages of the communities you wish to reach. Ensure that all imagery is inclusive and representative.
- Reduce Logistical Barriers: Offer flexible scheduling (evenings/weekends), provide transportation stipends or ride-share vouchers, and leverage decentralized trial elements like home health visits to make participation more feasible for people with hourly jobs or caregiving responsibilities.
- Address Historical Mistrust: Acknowledge past research abuses and address mistrust head-on through transparent communication, community advisory boards, and ensuring the diversity of your own research staff.
- Train Staff: Ensure all site staff have received training in cultural competency and unconscious bias to provide a welcoming and respectful environment for all participants.
What are the key ethical principles in patient recruitment?
Ethical recruitment is the non-negotiable foundation of all clinical trial recruitment strategies. The core principles, derived from the Belmont Report, are:
- Respect for Persons: This principle asserts that individuals must be treated as autonomous agents and that persons with diminished autonomy are entitled to protection. In practice, this is upheld through a robust informed consent process. This process must be completely voluntary, free of coercion, and ensure the participant fully comprehends the study’s purpose, procedures, risks, and benefits before agreeing to participate.
- Beneficence: This principle involves two parts: (1) do not harm, and (2) maximize possible benefits and minimize possible harms. For recruitment, this means all materials must be truthful and balanced. They cannot overstate potential benefits or downplay risks. The study design itself must be sound to ensure any risks taken on by participants are justified by the potential for valuable knowledge.
- Justice: This principle demands the fair distribution of the benefits and burdens of research. It means that specific groups (e.g., vulnerable populations) should not be unfairly targeted to bear the burdens of research, nor should other groups be unfairly excluded from its potential benefits. This is the ethical underpinning for the imperative to improve diversity in clinical trials.
All recruitment materials, strategies, and compensation plans must be reviewed and approved by an Institutional Review Board (IRB) or Ethics Committee to ensure these principles are upheld and that the rights and welfare of research participants are protected.
Conclusion
Effective clinical trial recruitment strategies are vital for accelerating medical innovation. Relying on traditional methods alone leads to costly delays and slows the delivery of new treatments to patients.
The future of recruitment is a hybrid, data-driven approach that puts the patient at the center. By combining patient-centric protocol design, powerful digital outreach, and strong partnerships with HCPs and advocacy groups, we can find the right patients faster and more efficiently. This ensures that groundbreaking research is not stalled by recruitment bottlenecks.
At Lifebit, we see patient recruitment as a data challenge. Our next-generation federated platform is designed to enable secure, real-time access to diverse, real-world data, empowering researchers to identify and recruit patient cohorts that were previously hidden. By connecting disparate datasets, we help accelerate the entire clinical research lifecycle.
Ready to make your next study a resounding success? Learn more about Lifebit’s solutions for clinical research and find how we can help you accelerate your journey.

