Seamless Healthcare Why Data Integration Standards Matter

Why Healthcare Data Integration Standards Are the Foundation of Modern Medicine
Data integration standards healthcare are the rules that allow different health systems to share patient information safely and accurately. Without them, critical health data remains trapped in disconnected silos—creating dangerous gaps in patient care, blocking research breakthroughs, and preventing AI from changing medicine.
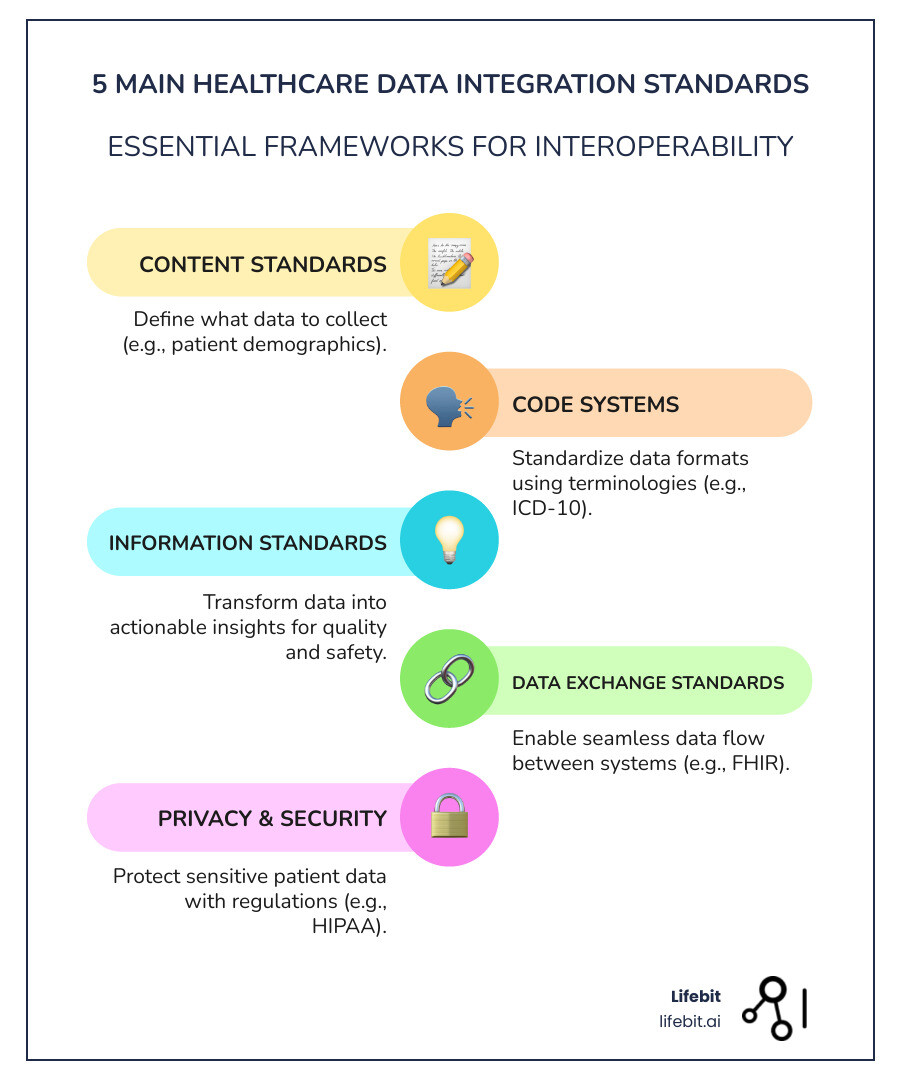
The key healthcare data integration standards include:
- Data Exchange Standards – HL7 FHIR, HL7v2, C-CDA (how data moves between systems)
- Terminology Standards – SNOMED CT, LOINC, RxNorm (what the data means)
- Core Data Standards – USCDI (what data must be shared in the US)
- Privacy Standards – HIPAA, ISO 27001 (how data is protected)
- Research Standards – CDISC (how trial data is structured)
The stakes are enormous. Healthcare generates 2,314 exabytes of data annually—30% of all data worldwide. Yet a single healthcare system can operate up to 18 different EHR platforms, each speaking its own language. When these systems can’t communicate, patients suffer. In 2023 alone, over 540 organizations reported data breaches affecting more than 112 million individuals.
Standards solve this chaos. They act as universal translators, turning fragmented records into a complete patient story. They enable real-time clinical decision support, power AI models that can predict sepsis hours before symptoms appear, and accelerate drug findy by connecting isolated research datasets.
As Maria Chatzou Dunford, CEO and Co-founder of Lifebit, I’ve spent over 15 years building platforms that break down barriers in biomedical data integration. I’ve helped pharmaceutical organizations and public health institutions use these standards to power secure, federated analytics at scale. Through this work, I’ve seen how the right standards transform data from a compliance burden into a strategic asset.
The Data Gridlock: Why Healthcare Is Drowning in Disconnected Information
Imagine a patient’s medical history scattered across a dozen different clinics, hospitals, and pharmacies. This isn’t hypothetical; it’s the daily reality of “data gridlock.” This fragmentation isn’t just inefficient—it’s dangerous. Consider a diabetic patient with a heart condition who visits their primary care physician, an endocrinologist, a cardiologist, and an urgent care clinic over six months. Without seamless data integration, the cardiologist might not see the new medication prescribed by the endocrinologist, increasing the risk of an adverse drug interaction. The urgent care physician, treating an acute issue, may order a redundant and expensive CT scan because they cannot access one done a month prior. These information gaps lead directly to duplicated tests, delayed diagnoses, medication errors, and ineffective treatment plans. Studies have estimated that the cost of repeated medical tests due to a lack of interoperability runs into billions of dollars annually in the U.S. alone.
The healthcare industry produces 30% of the world’s data, from EHRs and medical images to lab results and genomic data. While 96% of hospitals use EHRs, the problem isn’t a lack of data but a lack of connection. A single healthcare system can have up to 18 different EHR platforms, each a digital island. These data silos prevent systems from communicating. The challenge is compounded by the variety of data types. Structured data like lab results are easier to handle, but a vast amount of critical information is locked in unstructured formats like clinical notes, pathology reports, and discharge summaries. Integrating complex data types like high-resolution medical images (DICOM) and vast genomic sequences (VCF files) presents its own unique set of technical hurdles.
Beyond technical issues, data privacy and security are enormous challenges. Healthcare data is highly sensitive and subject to strict regulations like HIPAA in the USA, various provincial laws in Canada, and the GDPR in Europe. In 2023, over 540 organizations reported healthcare data breaches affecting more than 112 million individuals. Integrating data requires robust security to protect patient confidentiality and prevent breaches.
The administrative burden is also immense. Clinicians waste valuable time—sometimes hours per day—manually entering or searching for information across multiple systems. This not only drives up operational costs but is a major contributor to physician burnout and staff frustration.
These challenges lead to inefficient care coordination, increased costs, and suboptimal outcomes. The global healthcare data integration market is projected to grow at a CAGR of 14.5% through 2032, reflecting the urgent need to overcome these obstacles. Overcoming them requires a strategic approach focused on standardization and robust security. Without it, the promise of integrated, patient-centric care remains out of reach.
The Universal Translators: A Guide to Key Healthcare Data Integration Standards
In the chaotic world of healthcare data, standards act as “universal translators,” enabling disparate systems to speak the same language. They are the backbone of interoperability, facilitating both data exchange (moving information) and semantic interoperability (ensuring the information’s meaning is understood). These standards govern how data moves, what it means, and what must be shared.
Data Exchange Standards: The Highways for Health Information (HL7, FHIR)
Data exchange standards are the “highways” that allow patient data to travel between systems.
HL7 (Health Level Seven) International has long dominated this space, providing frameworks for exchanging electronic health information.
- HL7v2: The most widely adopted messaging standard, used by over 90% of U.S. healthcare organizations. It uses a flexible, event-driven protocol where messages are triggered by events like a patient admission (ADT^A01) or a new lab result (ORU^R01). However, its flexibility often led to custom implementations that hindered true interoperability. An HL7v2 message is a text-based string using pipes (|) and carets (^) as delimiters, which can be difficult to parse and requires specialized knowledge. For example, a patient name might look like
PID|||12345^^^MRN^MR||DOE^JOHN^A^^^||... - C-CDA (Consolidated Clinical Document Architecture): An HL7 standard for sharing clinical documents like discharge summaries and referral notes. It uses XML to structure entire documents, providing a human-readable snapshot of a patient’s care episode, but it is less suited for querying specific, granular data points in real time.
FHIR (Fast Healthcare Interoperability Resources): Launched by HL7 in 2014, FHIR is the modern API for real-time healthcare data. If HL7v2 is like sending a letter, FHIR is like an instant message. It uses web-based, developer-friendly RESTful APIs, making it far easier to integrate with modern applications, including mobile apps and cloud platforms.
FHIR’s modular “resources” (e.g., Patient, Observation, Medication, Encounter) are the fundamental building blocks. Each resource is a self-contained packet of information with a defined structure, expressed in modern formats like JSON or XML. This modularity is ideal for mobile and cloud communication, allowing developers to request specific data points (e.g., just the patient’s active allergies) instead of entire documents. This leads to faster, more efficient data exchange. To ensure consistency for specific use cases, the FHIR community develops Implementation Guides (IGs). These are published sets of rules and extensions that constrain the base FHIR specification, such as the Da Vinci Project IGs for payer-provider workflows or the CARIN Alliance IG for consumer-facing applications.
FHIR is rapidly becoming the global standard, with adoption in Israel, Singapore, and across Europe. We encourage you to learn more about FHIR and its transformative potential.
Here’s a quick comparison of HL7v2 and FHIR:
| Feature | HL7v2 | FHIR |
|---|---|---|
| Analogy | Sending a letter | Instant message |
| Data Structure | Pipe-and-caret delimited messages | Resource-based (Patient, Observation, Medication, etc.) |
| Technology | TCP/IP sockets, custom interfaces | RESTful APIs, HTTP, XML/JSON |
| Ease of Use | Complex, often requires custom parsing | Easier for modern developers, web-friendly |
| Real-time | Can be near real-time, but often batch | Designed for real-time access and updates |
| Modularity | Less modular, messages contain many fields | Highly modular, resources can be combined as needed |
| Use Cases | Interface engines, legacy system integration | Mobile apps, cloud services, advanced analytics, rapid development |
We believe FHIR is reshaping health tech by offering a more agile and efficient way to achieve interoperability.
Other critical exchange standards include:
- DICOM (Digital Imaging and Communications in Medicine): The universal standard for medical imaging (X-rays, MRIs, CT scans), handling not just the image data but also related metadata like patient ID and acquisition parameters.
- X12: Used in the US for administrative and financial transactions like electronic claims, eligibility inquiries, and remittance advice.
- SCRIPT: An NCPDP standard for e-prescribing, enabling the secure electronic transmission of prescriptions between prescribers and pharmacies.
Terminology Standards: Ensuring Everyone Speaks the Same Medical Language
While exchange standards handle how data moves, terminology standards address what it means. They ensure semantic interoperability, so “heart attack” in one system is understood as “myocardial infarction” in another. This is crucial for patient safety and reliable analytics.
- SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms): The most comprehensive clinical terminology, with over 350,000 concepts. Its power lies in its hierarchical structure and ability to define relationships between concepts. For example, “viral pneumonia” is a child of “pneumonia” and has a causative agent of “virus.” This allows for sophisticated querying and reasoning.
- LOINC (Logical Observation Identifiers Names and Codes): Provides universal codes for lab tests and clinical observations. This ensures a result for a specific test means the same thing everywhere. For example, LOINC code
2951-2uniquely identifies a “Sodium (serum or plasma)” measurement, regardless of the local lab’s name for the test. - RxNorm: A standardized nomenclature for clinical drugs in the US. It links different representations of the same drug—brand names (Lipitor), generic names (Atorvastatin), ingredients, and dose forms—to a single concept unique identifier (RxCUI). This is essential for accurate medication reconciliation and preventing adverse drug interactions.
- ICD (International Classification of Diseases): Used globally for classifying diseases for administrative and epidemiological purposes (e.g., ICD-10-CM for diagnosis codes in the US). It is primarily used for billing and population health statistics.
- CPT (Current Procedural Terminology): Used in the US to describe medical, surgical, and diagnostic services for billing and reimbursement.
Terminology mapping—linking local, proprietary codes to these standard terminologies—is a complex but vital process. Without it, the meaning of integrated data can be lost, leading to clinical errors and flawed analytics.
The Blueprint for US Interoperability: Understanding USCDI
In the United States, the USCDI (United States Core Data for Interoperability) is pivotal. Mandated by the 21st Century Cures Act, USCDI defines a standard set of health data classes and elements that certified health IT systems must be able to share via APIs.
Managed by the ONC, USCDI ensures a core set of patient information is consistently available. The standard is versioned and expands over time. For example, USCDI v1 included foundational data like Allergies, Medications, Clinical Notes, and Lab Results. Later versions (v2, v3, and v4) have expanded to include crucial data like Health Status, Encounter Information, Provenance (who created the data and when), and Social Determinants of Health (SDOH). This acknowledges that non-clinical factors are critical for holistic care. USCDI gives providers a more complete patient picture, forces vendors to compete on innovation rather than data blocking, and gives patients unprecedented access to their health information. Initiatives like USCDI+ for Public Health extend these benefits by defining data sets for specific public health reporting needs, such as for cancer or infectious disease surveillance.
From Standards to Breakthroughs: Powering AI, Research, and Better Patient Outcomes

The true power of data integration standards healthcare is turning raw information into actionable intelligence that revolutionizes patient care, accelerates research, and fuels AI.
Standardized, integrated data is a goldmine for AI and advanced analytics, opening up insights hidden in disconnected systems. Predictive models can identify patients at risk for sepsis hours in advance, forecast hospital readmission risk, or stratify populations for targeted outreach. Population health analytics can pinpoint at-risk communities for public health interventions, and generative AI can summarize lengthy clinical notes into structured FHIR resources, saving clinicians valuable time.
Standardized data also fuels groundbreaking research. Standards from CDISC (Clinical Data Interchange Standards Consortium) ensure consistency in clinical trial data, accelerating drug development and regulatory review. We adhere to these CDISC standards for clinical research, as they are crucial for global biopharma research. Integrating EHR data with Real-World Data (RWD) from sources like wearables, claims data, and patient registries generates Real-World Evidence (RWE). RWE can support regulatory decisions, create synthetic or external control arms for clinical trials (reducing the need for placebo groups), and conduct post-market safety monitoring to track a drug’s long-term effects.
Our federated AI platform leverages these standards to enable secure access to global biomedical data. We empower biopharma and public health agencies across the UK, USA, Europe, Canada, Israel, and Singapore to conduct large-scale research using federated learning. This allows AI models to train on distributed data without moving it, preserving privacy while accelerating research and maintaining the highest security standards.
Best practices for implementing data integration standards in healthcare
Implementing data integration standards healthcare is an ongoing journey, not a one-time project. Here are some best practices we advocate for:
- Define Clear Data Integration Objectives: Before choosing a solution, articulate what you aim to achieve. Is the primary goal improving clinical care coordination, enabling research capabilities, streamlining billing, or meeting regulatory requirements? Clear objectives will guide every subsequent decision.
- Conduct a Thorough Data Audit: Understand your existing data landscape—what data types you have, where they are stored, their formats, and their current quality. This “Know Your Data” step is foundational and should include data profiling to assess completeness, accuracy, and conformity.
- Select Appropriate Standards: Choose standards that align with your goals and regulatory environment. FHIR is the go-to for modern, real-time data exchange, while SNOMED CT and LOINC are essential for achieving semantic interoperability in clinical data. Don’t forget standards like DICOM for imaging and X12 for financial data.
- Implement a Scalable and Flexible Architecture: Leverage cloud-native architecture and APIs for flexibility and scalability. While traditional ETL (Extract, Transform, Load) processes are common for batch integrations, modern approaches also use real-time streaming (e.g., via Kafka) and consider architectures like data fabrics or data mesh to handle decentralized data sources effectively.
- Ensure Robust Security and Governance: Comply with strict data privacy regulations like HIPAA in the USA, provincial laws in Canada (overseen by bodies like the IPC in Ontario), and GDPR in Europe. Implement strong security controls, including encryption in transit and at rest, role-based access control (RBAC), and comprehensive audit trails. Establish a data governance framework to maintain data quality, security, and compliance over time.
- Train Your Team on FHIR Standards (and others): Your people are key to success. Empower your development, clinical, and informatics teams by providing comprehensive training on the standards you adopt. This fosters a culture of interoperability and ensures solutions are built correctly from the ground up.
- Use Analytics Tools to Generate Insights: Integration is the means, not the end. Once data is integrated and standardized, use modern analytics and business intelligence tools to interpret it and generate insights that support everything from daily operations to long-term strategic clinical decisions.
The future outlook for data integration standards in healthcare
The future of data integration standards healthcare is dynamic and increasingly intelligent. A key trend is the widespread adoption of FHIR. However, while FHIR excels at one-to-one data exchange, it wasn’t built for complex, population-level analytics. This has led to the development of complementary standards like the FHIR Bulk Data Access (Flat FHIR) API, which allows for the efficient export of large datasets for analytics. This gap is also being filled by platforms that transform FHIR data for large-scale analytics and AI/ML models, like our Trusted Data Lakehouse (TDL).
AI will also play a larger role in data management itself, automating tedious tasks like data cleansing, terminology mapping, and quality checks. Federated learning is gaining traction as a privacy-preserving technique that allows AI models to learn from decentralized data without centralizing it, addressing privacy concerns while deriving insights from distributed data pools.
Global standards initiatives are gaining momentum. The European Health Data Space Regulation (EHDS) aims to create a single market for health data in the EU, mandating FHIR for data exchange. In Canada, the Canadian Institute for Health Information (CIHI) leads national standards development. Emerging standards for genomics, like those from the Global Alliance for Genomics and Health (GA4GH), are also critical for advancing personalized medicine. These efforts show a global recognition that interoperability is an international imperative.
Finally, patient-centric data models will become more prevalent, giving individuals greater control over their health information through APIs and applications, driving further innovation and empowering patients as active participants in their own care.
Frequently Asked Questions about Healthcare Data Standards
What is the difference between data exchange standards (like FHIR) and terminology standards (like SNOMED)?
This is a great question that gets to the heart of interoperability! Think of it this way:
- Data exchange standards like FHIR and HL7v2 are the “envelope and postal service.” They define the technical framework for how healthcare information is moved from one system to another. They dictate the structure, format (like XML or JSON), and communication protocols (like RESTful APIs) for sending data. They ensure the message gets from point A to point B efficiently.
- Terminology standards like SNOMED CT, LOINC, and RxNorm are the “shared language” inside that envelope. They provide the standardized medical vocabulary and codes that give meaning to the data being exchanged. They ensure that when a system receives a message, it understands the clinical concepts, lab tests, or medications precisely and unambiguously.
Both are absolutely essential for true interoperability. You can send data all day long, but if the receiving system doesn’t understand what it means, it’s just noise.
Why are there so many different healthcare data standards?
It can certainly seem overwhelming! The proliferation of healthcare data standards is largely due to historical evolution and the specialized nature of different healthcare domains. Standards emerged over decades to solve specific problems in specific areas:
- DICOM was developed for the unique needs of medical imaging.
- HL7v2 arose in the early days of electronic health records to facilitate basic messaging between systems.
- X12 was created for administrative and financial transactions.
- SNOMED CT and LOINC address the need for universal clinical and laboratory terminologies.
Each standard addressed a particular need at a particular time. While this led to fragmentation, modern standards like FHIR are designed to act as a unifying layer, making it easier for these diverse systems and standards to communicate, much like a universal adapter for different types of plugs.
Who enforces the use of these standards?
Enforcement varies by region and by the specific standard. In the United States, key enforcers include:
- The Office of the National Coordinator for Health Information Technology (ONC): This is the primary body, especially through mandates stemming from the 21st Century Cures Act. The ONC defines standards like USCDI that certified health IT systems must adhere to to promote interoperability.
- Centers for Medicare & Medicaid Services (CMS): CMS implements policies that incentivize or require the use of specific standards, particularly for programs like Medicare and Medicaid, to ensure data exchange between payers, providers, and patients. Their interoperability policies began to go into effect in 2021.
- The Food and Drug Administration (FDA): For clinical research, the FDA enforces standards such as CDISC for the submission of clinical trial data.
- HIPAA (Health Insurance Portability and Accountability Act): While not a data content standard itself, HIPAA mandates privacy and security standards for protected health information (PHI), indirectly driving the need for secure data integration and exchange.
In Canada, organizations like CIHI play a leading role in developing and promoting national data standards, often in collaboration with provincial governments and Canada Health Infoway. Provincial bodies, such as the Information and Privacy Commissioner (IPC) in Ontario, oversee access and privacy laws, which influence how data integration is conducted.
In Europe, the upcoming European Health Data Space (EHDS) will establish a framework for health data exchange and will likely lead to greater standardization and enforcement across member states.
Conclusion: Turn Your Standardized Data into Actionable Intelligence
The journey towards seamless healthcare is fundamentally paved with data integration standards healthcare. These standards are not just technical requirements but essential tools for overcoming the data gridlock that plagues our healthcare systems. By acting as universal translators, standards like FHIR, SNOMED CT, and USCDI enable true interoperability, changing fragmented records into a comprehensive, patient-centric view.
This change is critical. It empowers clinicians with more effective care, reduces administrative burdens, and improves patient safety. Beyond the clinic, standardized data is the lifeblood of innovation. It fuels advanced analytics for predictive models and personalized medicine. It accelerates groundbreaking research, allowing us to generate Real-World Evidence and fast-track the development of new therapies.
The future of healthcare is integrated, intelligent, and proactive. At Lifebit, we are dedicated to building the platforms that make this future a reality. Our next-generation federated AI platform is designed to harness the power of standardized data, breaking down silos while preserving privacy and sovereignty. We believe that by changing your data from a compliance burden into a strategic asset, we can collectively drive findies and ultimately improve health outcomes for everyone.
Ready to open up the full potential of your healthcare data? Explore how a Trusted Data Lakehouse can unify your data and turn it into actionable intelligence.


