How to Navigate the European Medicines Agency Website

European Medicines Agency: Navigate 2024 Easily
Why Understanding the European Medicines Agency Matters
The European Medicines Agency serves as Europe’s central hub for medicine regulation, protecting the health of over 450 million people across the EU and European Economic Area. This decentralized EU agency plays a critical role in evaluating, authorizing, and monitoring medicines for both human and veterinary use.
Key Facts About the European Medicines Agency:
- Established: 1995 (formerly known as EMEA until 2009)
- Location: Amsterdam, Netherlands (relocated from London post-Brexit in 2019)
- Staff: Approximately 900 employees
- Network: 4,500 experts across EU member states
- Budget: €478.4 million annually (2024)
- Mission: Foster scientific excellence in medicine evaluation and supervision
The EMA operates through a centralized procedure that allows companies to submit a single marketing authorization application valid across all EU member states. This streamlined approach is mandatory for innovative medicines, including those derived from biotechnology, treatments for serious diseases like cancer and HIV/AIDS, and medicines for rare conditions.
For pharmaceutical companies, regulatory bodies, and healthcare organizations working with European markets, understanding how to steer the EMA’s digital resources is essential for accessing critical information about medicine approvals, safety data, and regulatory guidance.
As Maria Chatzou Dunford, CEO of Lifebit with experience in computational biology and health-tech, I’ve worked closely with regulatory frameworks and understand how the European Medicines Agency impacts drug development and real-world evidence generation. This guide will help you efficiently find the information you need on their comprehensive website.
European medicines agency vocabulary:
Understanding the EMA: Who They Are and What They Do
The European Medicines Agency (EMA) stands as a cornerstone of public and animal health protection within the European Union. Its primary mission is to foster scientific excellence in the evaluation and supervision of medicines, ensuring that only safe, effective, and high-quality treatments reach patients. This commitment to scientific rigor and public well-being is at the heart of everything the agency does.
Our journey with the EMA begins in 1995, the year it was established. Originally known as the European Agency for the Evaluation of Medicinal Products (or EMEA), it underwent a significant renaming in December 2009 to become simply the European Medicines Agency – a change that reflected its maturing role and broader scope. Fast forward to March 2019, and the agency started on another major transition: its relocation from London to Amsterdam following Brexit. This move, while challenging, underscored the agency’s resilience and its continued dedication to its mandate within the EU.
Today, the EMA operates as a lean but powerful organization. With a dedicated staff of around 897 employees, the agency orchestrates a vast network of about 4,500 experts spread across the EU. These experts, often drawn from national competent authorities, are the scientific engine behind the EMA’s evaluations. Operating on an annual budget of €478.4 million in 2024, the EMA efficiently manages its responsibilities, from authorizing cutting-edge therapies to monitoring the safety of medicines throughout their lifecycle.
For anyone looking to dig deeper into the EMA’s foundational principles and operations, the Official EMA Website is your first and best stop.
Where to Find “About Us” and Strategic Plans
When you visit the EMA’s website, one of the most informative sections is “About Us.” This is where you can truly grasp the agency’s mission, its history, and its operational structure. We find that exploring this section provides invaluable context for understanding the EMA’s decisions and initiatives.
Within “About Us,” you’ll find details about the EMA’s Management Board, which oversees the agency’s strategic direction. You can also trace the EMA’s historical milestones, including its establishment and evolution. For those interested in the bigger picture, the website also houses key strategic documents. For instance, the ‘Road Map to 2010’ and the ‘2015 Road Map’ initiatives provide insights into the agency’s long-term vision and evolving priorities over the years. These documents outline how the EMA plans to adapt to new scientific challenges, technological advancements, and public health needs, guiding its efforts to continue fostering scientific excellence in medicine regulation. We leverage our expertise in Lifebit AI and Lifebit Trusted Research Environment to understand how such strategic plans can impact the future of data-driven medicine.
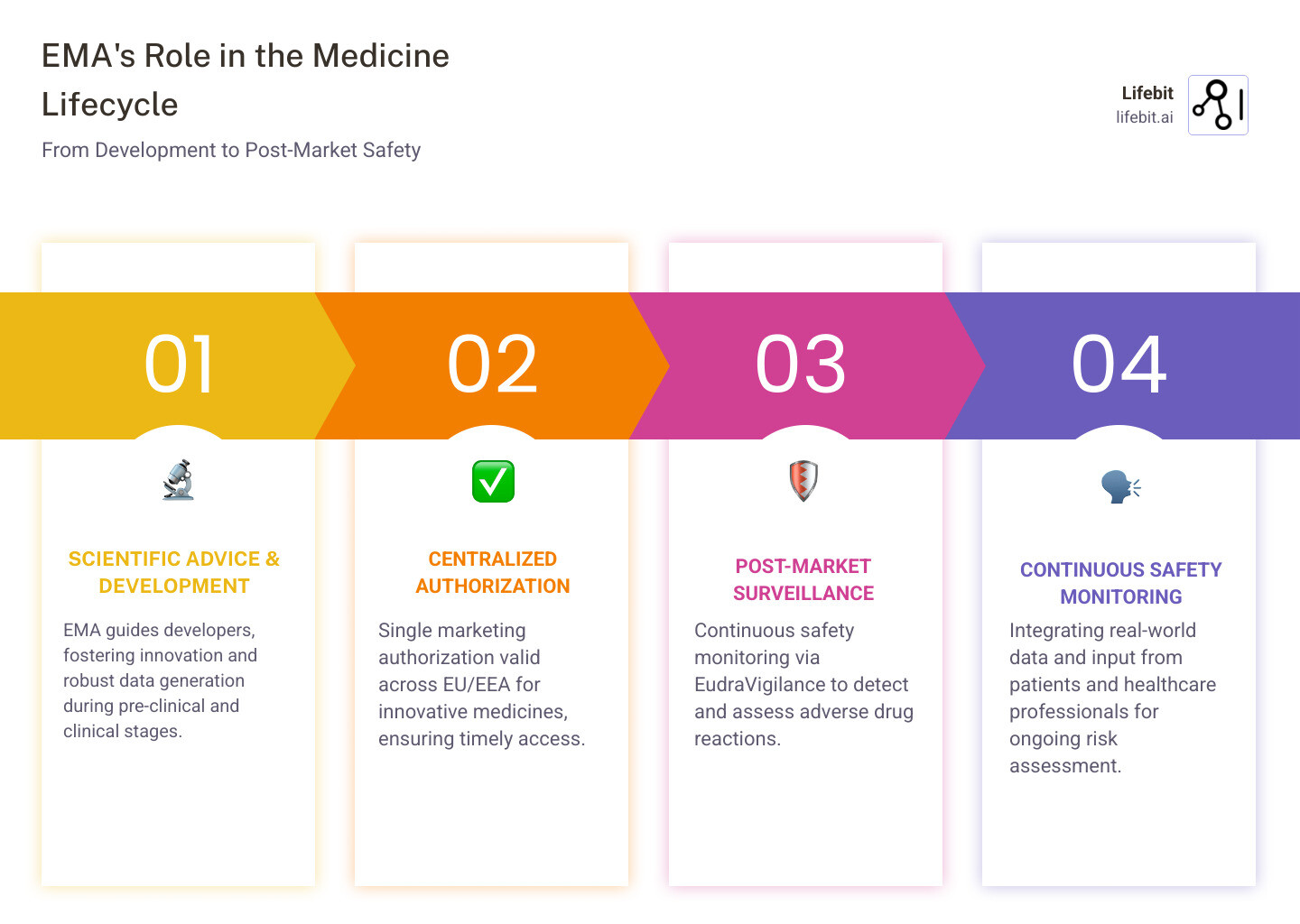
The Medicine Lifecycle: Finding Authorization and Development Information
When we think about how medicines reach patients, it’s helpful to understand that the European Medicines Agency doesn’t just approve drugs and walk away. Instead, they’re involved throughout the entire journey – from the moment a pharmaceutical company has a promising compound in the lab all the way through to monitoring how that medicine performs in the real world.
The EMA’s key responsibilities center around scientific evaluation and supervision of medicines. But here’s what makes them special: they’re not just gatekeepers saying “yes” or “no” to new treatments. They actively support innovation by providing scientific advice to developers and facilitating research that could lead to breakthrough therapies.
Think of it like having a knowledgeable mentor who helps guide you through a complex process while also making sure everything meets the highest safety standards.
How the european medicines agency Authorizes Medicines
One of the most impressive things about the European Medicines Agency is how they’ve streamlined the authorization process. Instead of forcing companies to knock on 30 different doors across Europe, the EMA offers a centralized procedure that’s remarkably efficient.
Here’s how it works: a pharmaceutical company submits one single marketing authorization application to the EMA. If approved, that authorization becomes valid across all EU and EEA member states – that’s 30 countries with one decision. Pretty smart, right?
The EMA aims for a 210-day approval timetable, though this can extend when they need additional information from applicants (what they call “clock stops”). This might sound like a long time, but considering the complexity of evaluating new medicines, it’s actually quite speedy.
Now, not every medicine goes through this centralized route – it’s actually mandatory for certain types of treatments. These include biotech medicines, new active substances for serious conditions like cancer, AIDS, and diabetes, and orphan medicines for rare diseases. This makes sense when you think about it – these are often the most innovative treatments that patients across Europe need access to quickly.
You can find all the details about this process by locating the ‘Authorisation of medicines’ section on the EMA website, where they break down exactly how this system protects patients while fostering innovation.
Special Programs for Rare Diseases and Children
The EMA has a heart for the underdog – specifically, patients with rare diseases and children who historically haven’t had many treatment options. These special programs show how the agency goes beyond basic drug approval to address real gaps in healthcare.
For rare diseases, there’s something called Orphan Designation. The Committee for Orphan Medicinal Products (COMP) handles these cases, offering incentives for rare disease treatments like fee reductions, scientific advice, and market exclusivity. When a disease affects fewer than 5 in 10,000 people in the EU, companies get extra support to develop treatments that might not otherwise be profitable.
Children get similar attention through the Paediatric Regulation. The Paediatric Committee (PDCO) evaluates Paediatric Investigation Plans (PIPs) – detailed roadmaps for how medicines will be studied in children. This ensures kids aren’t just getting adult medicines in smaller doses, but treatments specifically tested for their age groups.
These programs highlight something important: the EMA recognizes that one-size-fits-all doesn’t work in medicine. They’ve created pathways to ensure even the smallest patient populations get the innovative treatments they deserve.
At Lifebit, we understand how complex data needs can be for these specialized clinical trials, which is why our federated platform is designed to support the intricate research requirements these programs demand. More info about clinical trial data solutions.
Post-Market Safety: How to Access Pharmacovigilance Data
When a medicine gets the green light from the European Medicines Agency, that’s really just the beginning of its safety story. The real work of keeping patients safe continues long after authorization through what’s called lifecycle monitoring. Think of it as having a dedicated team of medicine detectives who never clock out!
This continuous safety monitoring during the post-authorisation phase is known as pharmacovigilance. It’s all about detecting, assessing, understanding, and preventing adverse effects or any other medicine-related problems that might pop up once a treatment is being used by real patients in the real world.
The crown jewel of this safety surveillance system is the EudraVigilance database. This centralized European database is like a massive digital filing cabinet that collects suspected adverse reactions to medicines. Whether a treatment is already authorized or still being studied in clinical trials within the European Economic Area, any suspected side effects get logged here. It’s an incredible repository of safety information that helps identify potential warning signs early – sometimes even before patterns become obvious to individual doctors or patients.

Using the european medicines agency Database for Adverse Drug Reactions
Accessing the EudraVigilance database is surprisingly straightforward, whether you’re a healthcare professional, a concerned patient, or just someone who likes to dig into the data. You can search for suspected side effects related to specific medicines, which can be incredibly valuable for understanding potential risks before starting a new treatment.
Now, interpreting these reports isn’t always simple – it requires understanding that correlation doesn’t equal causation. Just because someone experienced a side effect while taking a medicine doesn’t automatically mean the medicine caused it. The European Medicines Agency provides helpful guidance on how to read these reports with the right context and nuance.
What makes this database truly special is its commitment to transparency. The EMA believes that patients and healthcare professionals deserve access to safety information, not just regulatory agencies behind closed doors. We encourage you to Check the European suspected adverse drug reactions database to explore this valuable resource yourself.
Behind the scenes, there’s a dedicated group of experts working hard to make sense of all this safety data. The Pharmacovigilance Risk Assessment Committee (PRAC) serves as the brain trust for evaluating safety signals. This expert committee doesn’t just look at EudraVigilance data – they pull information from multiple sources to get the complete picture of a medicine’s risk assessment and management profile.
When PRAC spots a potential safety concern, they don’t just file a report and move on. They dig deeper, analyze the evidence, and make concrete recommendations to ensure medicines remain safe for patients. Sometimes this means updating a medicine’s labeling, sometimes it means requiring additional studies, and occasionally it might mean removing a medicine from the market entirely.
The insights that come from this continuous monitoring of real-world safety data are absolutely crucial. They don’t just protect current patients – they also inform how future medicines are developed and tested. At Lifebit, we understand how this kind of real-world evidence can transform pharmacovigilance, helping spot safety signals faster and more accurately through advanced analytics and AI-driven surveillance systems.
For Professionals and Partners: Committees, Collaborations, and Careers
Working with the European Medicines Agency means tapping into one of the world’s most sophisticated regulatory networks. Think of it as a scientific ecosystem where brilliant minds collaborate to keep medicines safe and effective. The EMA’s scientific committees form the backbone of this work, bringing together top experts from across Europe who dedicate their expertise to protecting public health.
What makes the EMA truly remarkable is how it thinks globally while acting locally. The agency doesn’t operate in isolation – it actively partners with major organizations like the World Health Organization (WHO) and the U.S. Food and Drug Administration (FDA). These collaborations happen through frameworks like the International Council for Harmonisation (ICH), which helps align regulatory standards worldwide. This global approach means that when a medicine gets approved, it’s been evaluated against internationally recognized standards.
The EMA also excels at stakeholder engagement. Whether you’re a patient trying to understand your treatment options, a healthcare professional seeking regulatory guidance, or a pharmaceutical company navigating complex approval processes, the agency works hard to provide clear, accessible information. They regularly hold bilateral meetings with industry groups like Vaccines Europe, discussing everything from submission timelines to public health emergency preparedness. It’s this kind of open dialogue that keeps the regulatory process both rigorous and practical.
For those working in biomedical research and data analytics, like we do at Lifebit, understanding these collaborative networks is essential. The data standards and regulatory frameworks these committees establish directly impact how we approach Clinical Trials, Genomics, and Data Security in our federated research environments.
Key Committees and Their Roles
Getting to know the EMA’s committees is like meeting the different departments of a world-class research university. Each committee brings specialized expertise to the table, and together they create a comprehensive evaluation system that few regulatory agencies can match.
The Committee for Medicinal Products for Human Use (CHMP) sits at the heart of medicine evaluation. This powerhouse committee handles marketing authorization applications for human medicines, and in 2024 alone, they recommended 13 new medicines for approval across the EU. You can dive deeper into their work at Committee for Medicinal Products Human Use (CHMP).
For our four-legged friends, the Committee for Medicinal Products for Veterinary Use (CVMP) ensures that animal medicines meet the same rigorous standards. Their work impacts everything from pet medications to treatments for livestock, making sure that veterinary care remains safe and effective. You can explore their activities at Committee for Medicinal Products Veterinary Use (CVMP).
The Pharmacovigilance Risk Assessment Committee (PRAC) serves as the safety watchdog, continuously monitoring medicines after they reach the market. As we discussed earlier, they’re the experts who analyze data from EudraVigilance and recommend actions when safety concerns arise.
Perhaps the most exciting committee for the future of medicine is the Committee for Advanced Therapies (CAT). They handle cutting-edge treatments like gene therapies and cell-based medicines – the kind of treatments that seemed like science fiction just a few decades ago. Their work shapes how these groundbreaking therapies reach patients safely. Learn more at Committee for Advanced Therapies (CAT).
The Committee for Orphan Medicinal Products (COMP) focuses on rare diseases, ensuring that even the smallest patient populations get attention from drug developers. Meanwhile, the Paediatric Committee (PDCO) makes sure that children aren’t forgotten in medicine development – a historically overlooked area that’s now getting the attention it deserves.
What’s particularly valuable about the EMA’s transparency is that you can access meeting agendas, minutes, and scientific opinions for all these committees right on their website. It’s like having a front-row seat to the regulatory process. And if you’re inspired to join this important work, you can Find career opportunities at the EMA and contribute directly to European public health.
This collaborative approach to regulation, combined with robust Data Standardization, creates an environment where innovative research can thrive while maintaining the highest safety standards.
Frequently Asked Questions about the European Medicines Agency
How does the EMA compare to the US FDA?
It’s a question we hear often: how does the European Medicines Agency stack up against its American counterpart, the U.S. Food and Drug Administration (FDA)? While both agencies share the fundamental goal of ensuring medicine safety and efficacy, their approaches are quite different – and understanding these differences can help you steer both systems more effectively.
The most striking difference lies in how they’re organized. The European Medicines Agency operates as a decentralized network, coordinating the scientific expertise of national medicines agencies across all EU member states. Think of it like a well-orchestrated symphony, where the EMA conducts while national agencies contribute their specialized expertise. The FDA, in contrast, functions as a single federal body responsible for drug regulation across the entire United States.
This structural difference creates interesting variations in their approval processes. The EMA’s centralized procedure offers one marketing authorization that’s valid across all EU/EEA countries – essentially 30 countries with one decision. The FDA’s New Drug Application (NDA) process leads to approval for the entire U.S. market. While the EMA aims for a 210-day approval timeline, the FDA’s process can take longer, though direct comparisons get tricky due to different “clock stop” mechanisms and varying product types.
Despite these differences, both agencies work together through harmonization efforts, particularly via the International Council for Harmonisation (ICH). This collaboration helps align regulatory requirements globally, making it easier for companies to develop medicines that can reach patients worldwide.
How did the EMA respond to the COVID-19 pandemic?
The COVID-19 pandemic threw an unprecedented curveball at global public health, and the European Medicines Agency responded with remarkable speed and flexibility. Their actions during this crisis really showcased how regulatory agencies can adapt without compromising safety.
The EMA quickly introduced rolling reviews for COVID-19 vaccines and treatments. Instead of waiting for complete application packages, they evaluated data from ongoing clinical trials as it became available. This meant continuous assessment and faster decision-making while maintaining the same rigorous scientific standards we expect.
They also used conditional marketing authorizations strategically. This approach allowed earlier access to promising medicines based on preliminary but promising data, with the understanding that companies would provide additional safety and efficacy information after approval. It was a calculated risk that paid off enormously for European patients.
Perhaps most importantly, the EMA maintained exceptional transparency throughout the pandemic. They provided regular updates on evaluations, decisions, and safety monitoring activities. This open communication was crucial for building public trust during uncertain times. Their swift actions helped ensure that safe and effective COVID-19 vaccines and treatments reached European citizens as quickly as possible, while never cutting corners on safety.
What information does the EMA provide for patients?
The European Medicines Agency recognizes that informed patients make better health decisions. That’s why their website offers a wealth of resources designed specifically for patients and their families – all written in language that real people can understand.
The crown jewel for patients is the European Public Assessment Reports (EPARs). These public summaries break down the scientific evaluation of centrally authorized medicines into clear, non-technical language. They explain why a medicine was approved, how it works, and how it should be used safely. No PhD required!
The EMA also provides valuable guidance on package leaflets – those sometimes overwhelming inserts that come with your medication. The agency actively works to make these leaflets clearer and more user-friendly, ensuring you can easily understand how to take your medication safely and what to watch out for.
Patients can also learn how to report suspected adverse reactions directly through the EMA’s systems. This means you can play an active role in medicine safety monitoring – your experience matters and contributes to keeping medicines safe for everyone.
The agency regularly conducts public consultations too, giving patients and patient organizations opportunities to share their perspectives on various regulatory topics. This ensures that the patient voice is heard loud and clear in the regulatory process, not just the voices of companies and healthcare professionals.
Conclusion
As we wrap up our journey through the European Medicines Agency website, it’s clear that this digital resource is much more than a bureaucratic portal. It’s a living, breathing hub that connects patients, healthcare professionals, and industry experts to the heart of European medicine regulation.
Throughout this guide, we’ve explored how the EMA serves as Europe’s guardian of medicine safety and efficacy. From the centralized authorization process that streamlines approvals across 30 countries, to the comprehensive pharmacovigilance systems that monitor drug safety long after approval, the agency’s work touches every aspect of the medicine lifecycle. Their dedicated support for innovation shines through in programs for rare diseases and pediatric medicines, ensuring that even the smallest patient populations aren’t forgotten.
The EMA’s central role in protecting European public health has evolved remarkably since its establishment in 1995. What started as EMEA has grown into a sophisticated network of nearly 900 staff and 4,500 experts, operating from Amsterdam with a clear mission: fostering scientific excellence in medicine evaluation and supervision.
But here’s where things get really exciting for the future. The regulatory landscape is undergoing a data revolution, and the European Medicines Agency is leading the charge. Real-world data (RWD) and real-world evidence (RWE) are no longer nice-to-have extras – they’re becoming essential tools for continuous safety monitoring and smarter regulatory decisions.
This shift toward data-driven regulation creates both opportunities and challenges. How do you securely analyze vast amounts of sensitive health data across multiple countries and institutions? How do you ensure compliance while enabling the kind of rapid insights that modern pharmacovigilance demands?
That’s exactly the challenge Lifebit was built to solve. Our federated platform enables secure analysis of real-world data for pharmacovigilance and research, perfectly aligning with the EMA’s forward-looking data strategies. By providing compliant, real-time access to global biomedical data, we’re helping regulatory bodies and pharmaceutical companies accelerate the discovery and development of life-changing treatments.
The future of medicine regulation isn’t just about following rules – it’s about using the power of data to make better, faster decisions that ultimately save lives. And that’s a future we’re proud to help build.

