GLP-1 Trials Explained: From Lab to Life
Why GLP-1 Clinical Trials Are Changing Obesity Treatment
GLP-1 clinical trials are revolutionizing how we treat obesity and diabetes, with new drugs delivering weight loss results that rival bariatric surgery.
Key GLP-1 Trial Results:
- Retatrutide: 24.2% weight loss (triple-agonist)
- Tirzepatide: 22.5% weight loss (dual-agonist)
- Oral Semaglutide: 17.4% weight loss (oral formulation)
- Orforglipron: 14.7% weight loss (daily pill, no food restrictions)
What Makes These Trials Different:
- Oral medications replacing daily injections
- Multi-hormone approaches targeting GLP-1, GIP, and glucagon receptors
- Bariatric surgery-level weight loss without surgery
- Improved safety profiles with better tolerability
While lifestyle interventions offer ~10% weight loss with regain and bariatric surgery delivers 25-30% but isn’t scalable, new GLP-1 receptor agonists hit the sweet spot of efficacy and accessibility.
Many people with type 2 diabetes still do not meet treatment goals, driving the need for new therapies. This has spurred innovation in incretin-based medicines, moving beyond simple GLP-1 receptor activation to multi-hormone strategies.
The most exciting development is oral formulations that eliminate the injection barrier. Orforglipron’s ATTAIN-2 trial showed participants lost an average of 10.5% body weight while reducing A1C by 1.8%—all with a once-daily pill taken anytime.
As CEO and Co-founder of Lifebit, with 15+ years in computational biology, I help pharmaceutical organizations analyze GLP-1 clinical trials data across secure, federated environments. My experience building precision medicine tools gives me unique insight into how these breakthrough trials are reshaping obesity treatment.
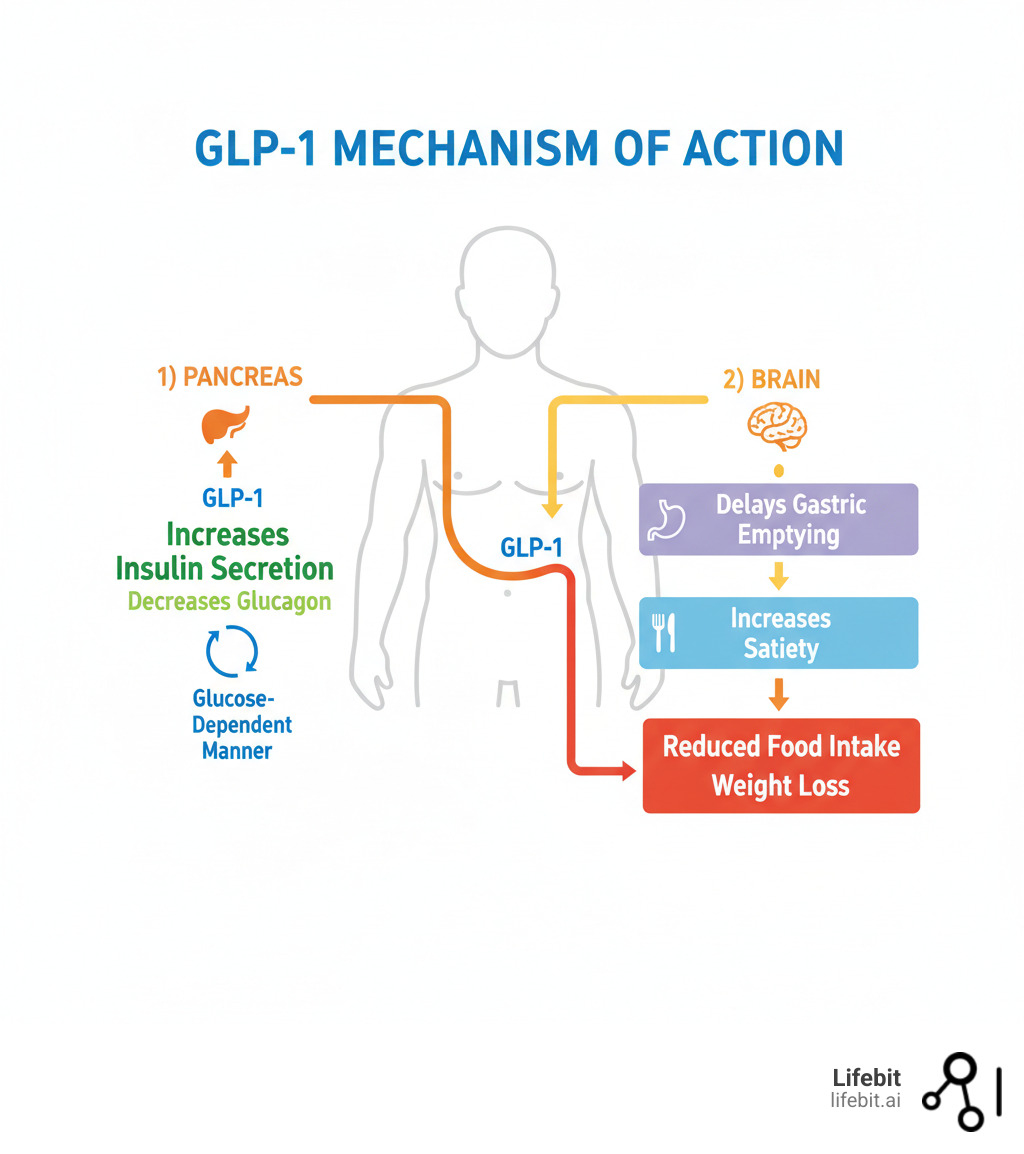
Quick look at GLP-1 clinical trials:
Lose the Needle, Not the Weight: The Oral GLP-1 Breakthrough
For many patients, effective GLP-1 medications have required daily or weekly injections, a real barrier to treatment. That’s changing fast. Oral GLP-1 medications are delivering impressive results without a single injection.
This isn’t just reformulation. Small-molecule drug innovation has created new nonpeptide agonists that survive digestion and are absorbed when swallowed, opening treatment to millions. Imagine managing weight and blood sugar with a daily pill—no injection anxiety, refrigeration, or disposal concerns. This convenience is key for long-term adherence.
Orforglipron: The Daily Pill Ready to Disrupt the Market
Lilly’s orforglipron delivered jaw-dropping results in the ATTAIN-2 trial. This daily pill led to an average loss of 22.9 pounds (10.5% of their body weight) over 72 weeks. Impressively, these participants had both obesity and type 2 diabetes, making weight loss more challenging.
The diabetes improvements were also striking: A1C levels dropped by 1.8% from 8.1%, and 75% on the highest dose reached the target A1C of 6.5% or less. Additionally, orforglipron reduced the inflammation marker high-sensitivity C-reactive protein by 50.6%, suggesting cardiovascular benefits.
How does it work? Orforglipron is a nonpeptide agonist. Unlike large protein-like GLP-1 drugs, this small molecule passes through the intestinal wall intact. In the bloodstream, it activates GLP-1 receptors to control insulin, slow digestion, and signal fullness. Scientific research on orforglipron’s mechanism reveals how this works.
Its safety profile is similar to other GLP-1 drugs, with GI side effects (nausea, vomiting, diarrhea) being the main concern. These were mostly mild to moderate and improved over time. Dropout rates were 6-11% due to side effects, versus 5% on placebo, meaning about 90% of people continued treatment.
Lilly plans global regulatory submissions this year. If approved, orforglipron could be the first widely available oral GLP-1 for obesity.
The Oral Competition: Semaglutide and Danuglipron
Orforglipron isn’t the only oral player in GLP-1 clinical trials. The race is heating up.
Oral semaglutide (high-dose) showed remarkable results in the OASIS-1 trial, where people without diabetes lost 17.4% of their body weight over 68 weeks. For those with diabetes, the PIONEER PLUS trial showed 9.8% weight loss and A1C reductions up to 2.1%. A notable side effect was “altered skin sensation” with the 50mg dose, which is being monitored.
Danuglipron showed promise with 11.7% weight loss after 32 weeks in Phase 2b results, but its twice-daily dosing created tolerability issues, leading to program adjustments. This highlights the challenge of balancing efficacy and tolerability in oral GLP-1 development.
The oral revolution is just beginning, removing the injection barrier and opening doors for millions who need effective obesity and diabetes management.
Beyond Single-Hormone Hits: How Multi-Agonists Are Delivering Bariatric-Level Weight Loss
Obesity treatment is evolving beyond the single-hormone approach. While GLP-1 receptor agonists are game-changers, scientists found that combining multiple gut hormones creates a synergistic effect more powerful than any single hormone. These multi-agonist therapies target GLP-1, GIP (Glucose-dependent Insulinotropic Polypeptide), and glucagon receptors.

GIP influences fat storage and burning, not just insulin. Glucagon, when combined with GLP-1, surprisingly increases energy expenditure and suppresses appetite. These multi-agonist approaches are delivering weight loss results that rival bariatric surgery (20-25%), something once thought impossible with medication alone.
Retatrutide (Triple G): The “King Kong” of Weight Loss Drugs?
Retatrutide, a triple-hormone-receptor agonist from Eli Lilly, targets GIP, GLP-1, and glucagon receptors. The results from its GLP-1 clinical trials are stunning.
In its 48-week Phase 2 trial, participants with obesity achieved a mean weight reduction of 24.2% at the highest dose. Every single participant on the 8 mg or 12 mg doses lost at least 5% of their body weight, and 36-48% lost 25% or more—bariatric surgery-level results from a weekly injection.
Beyond weight loss, 72% of participants with prediabetes returned to normal blood sugar levels, compared to just 22% in the placebo group.
However, GI side effects were more pronounced, affecting 73-94% of participants, with discontinuation rates reaching 13% due to adverse events. Dose-dependent heart rate increases were also observed, though they peaked at 24 weeks and then declined. The retatrutide trial represents a pivotal moment, with medications delivering surgical-level results without surgery.
Tirzepatide (Dual-Agonist): The First “Twincretin”
Before retatrutide, the dual-agonist tirzepatide was already rewriting the playbook by targeting both GLP-1 and GIP receptors. The SURMOUNT trial program has consistently shown its impact.
The SURMOUNT-1 trial delivered 22.5% weight loss over 72 weeks for people without diabetes. For those with both obesity and type 2 diabetes, SURMOUNT-2 showed 15.7% weight loss.
Critically, the SURMOUNT-4 trial showed that when people stopped taking tirzepatide, they regained an average of 14% of their body weight. Those who continued treatment lost an additional 5.5%. This underscores that obesity is a chronic disease requiring chronic treatment.
Tirzepatide’s safety profile is encouraging, with only 4-7% of participants discontinuing due to adverse events. Its success proved that targeting multiple hormone pathways simultaneously was revolutionary.
Unpacking the Data: A Deep Dive into GLP-1 Clinical Trials
Comparing the impressive weight loss numbers from GLP-1 clinical trials requires context. Each trial is unique, with different patient populations, durations, and endpoints, making direct comparisons tricky. For example, a 72-week trial provides more meaningful data on long-term weight maintenance than a 24-week study.
| Drug/Agonist Type | Target Population | Max Mean Weight Loss (%) | Max A1C Reduction (%) | Common GI Side Effects (Nausea/Vomiting/Diarrhea) | Discontinuation due to AEs (%) | Trial Duration |
|---|---|---|---|---|---|---|
| Retatrutide (GIP/GLP-1/GCG) | Obesity (w/ or w/o T2D) | 24.2% (Obesity) | 2.2% (T2D) | High (73-94% overall AEs) | 13% | 48 weeks |
| Tirzepatide (GIP/GLP-1) | Obesity (w/ or w/o T2D) | 22.5% (Obesity) | 2.1% (T2D) | Moderate (4-7% discontinuation) | 4-7% | 72 weeks |
| Oral Semaglutide 50mg (GLP-1) | Obesity (w/o T2D) | 17.4% | N/A (Obesity w/o T2D) | Common | Not specified (OASIS-1) | 68 weeks |
| Oral Semaglutide 50mg (GLP-1) | T2D | 9.8% | 2.1% | Common | Not specified (PIONEER PLUS) | 68 weeks |
| Orforglipron (GLP-1) | Obesity/Overweight & T2D | 10.5% | 1.8% | Common (20-36% Nausea) | 10.6% | 72 weeks |
| Survodutide (GLP-1/GCG) | Obesity | 18.7% | N/A | Common | Not specified | 46 weeks |
| Pemvidutide (GLP-1/GCG) | Obesity | 15.6% | N/A | Common | Not specified | 48 weeks |
| Efinopegdutide (GLP-1/Glucagon) | Obesity | 11.8% | N/A | Common | Not specified | 26 weeks |
| Mazdutide (GLP-1/GCG) | Overweight/Obesity | 15.4% | 1.7% (T2D) | Common | Not specified | 24 weeks |
| Danuglipron (GLP-1) | Obesity & T2D | 11.7% | 1.2% | Common | Not specified | 32 weeks |
Note: Weight loss percentages are maximum observed in respective trials. A1C reductions are maximum observed in T2D populations. “Common GI Side Effects” indicates they were frequently reported.
Efficacy in GLP-1 clinical trials: Who Wins the Weight Loss Race?
The numbers are stunning. Retatrutide leads with 24.2% weight loss in 48 weeks—bariatric surgery-level results. Tirzepatide follows at 22.5% over a longer 72-week period.
The oral medications are also competitive. Oral semaglutide hit 17.4% in people without diabetes, while orforglipron achieved 14.7% in a more challenging population with both obesity and type 2 diabetes.
Newer dual GLP-1/glucagon agonists are also impressive: survodutide showed 18.7% weight loss, pemvidutide reached 15.6%, and mazdutide delivered 15.4%. These therapies are doubling or tripling the 5-10% weight loss from traditional lifestyle interventions.
These results come from different patient populations and trial lengths. Retatrutide’s 24.2% was in people with obesity but without diabetes, while Orforglipron’s 14.7% was achieved in people with both conditions—a harder clinical challenge.
Safety and Tolerability in GLP-1 clinical trials: The Price of Potency
Higher efficacy often comes with predictable side effects. Across all GLP-1 clinical trials, nausea, vomiting, and diarrhea are the most common complaints as the digestive system adjusts. Fortunately, most side effects are mild to moderate and improve over time, especially with careful dose titration.
Tirzepatide shows the best tolerability, with only 4-7% of people discontinuing treatment. Retatrutide saw 13% discontinuation, which is reasonable given its potent metabolic effects. Orforglipron sits at 10.6% discontinuation, aided by a smart dose-escalation strategy.
Beyond GI effects, secondary benefits are emerging. Orforglipron reduced inflammatory markers by 50.6%, and A1C reductions of 1.8-2.2% are clinically meaningful. Heart rate increases seen with some multi-agonists like retatrutide are typically transient. The bottom line is that the safety profiles are manageable for most people with proper medical supervision.
What’s Next? The Future of Hormone-Based Obesity Drugs
The success of GLP-1 clinical trials is rapidly advancing obesity treatment, with drugs now delivering bariatric surgery-level weight loss without surgery. Researchers are pushing beyond single-target approaches, combining hormones and exploring new pathways to broaden treatment effectiveness.
The Expanding Pipeline: From Amylin to GIPR Antagonists
The drug pipeline is packed with innovative approaches beyond traditional GLP-1 agonists.
GLP-1/Glucagon combinations show incredible promise. Survodutide delivered up to 18.7% weight loss over 46 weeks and outperformed semaglutide in a head-to-head trial in people with type 2 diabetes. Pemvidutide, another combo, achieved 15.6% weight loss at 48 weeks. Efinopegdutide achieved 11.8% weight loss and significantly reduced liver fat by 72.7% in people with fatty liver disease.
Amylin is making a comeback. This hormone, which helps control appetite, is being combined with semaglutide in a drug called cagrisema to boost weight loss.
Interestingly, GIPR antagonists offer a different approach. While most drugs activate the GIP receptor, AMG133 blocks it while activating GLP-1, showing 14.5% weight loss by day 85. This highlights the ongoing GIP agonist vs. antagonist debate. Scientists are also exploring completely new pathways like Growth Differentiation Factor 15 (GDF-15) agonists.
The Ultimate Goal: Personalized and Sustainable Obesity Management
The real game-changer is treating obesity as the serious chronic disease it is, moving past simplistic advice that ignores complex biological factors. These new treatments prove obesity has biological roots that respond to medical intervention. Understanding the complications of obesity shows why this shift is so important.
The reality is that most people regain weight when they stop these medications. This is biology, not a failure of willpower, underscoring the need for long-term therapy.
The future is exciting. Combination therapies will allow for personalized medicine, using a person’s unique profile to predict the best treatment. We’re also moving toward holistic support systems that combine medication with nutrition, exercise, and behavioral support.
The role of GLP-1 clinical trials will continue to grow as researchers refine these approaches and gather long-term safety data. The field is moving incredibly fast, and the next five years promise even more breakthroughs.
Conclusion: Turning Trial Data into Real-World Cures
The evolution of GLP-1 clinical trials is a landmark in modern medicine. We’ve shifted from surgery or marginal results to oral medications delivering up to 24.2% weight loss, rivaling invasive procedures. For millions struggling with obesity and related conditions, these breakthrough treatments are life-changing.
Oral GLP-1s are democratizing access to care. The convenience of a daily pill like orforglipron, which delivered 10.5% weight loss, represents a massive leap in patient accessibility. Meanwhile, multi-agonists are pushing the boundaries of what’s medically possible, with drugs like retatrutide delivering weight loss comparable to bariatric surgery. This is a complete paradigm shift in treating obesity as a chronic disease.
However, these results are meaningless if they don’t reach patients. This is where advanced data analytics is critical. Modern GLP-1 clinical trials generate vast, complex data. Analyzing it quickly and securely is essential for bringing treatments to market safely and efficiently.
This is why federated AI platforms like ours exist. We help pharmaceutical organizations analyze trial data across secure, distributed environments without compromising patient privacy. Our platform enables researchers to spot safety signals faster, identify promising patient populations, and accelerate the journey from trial results to approved therapies.
With 15+ years in computational biology, I’ve seen how the right data infrastructure accelerates medical breakthroughs. The GLP-1 clinical trials delivering these results rely on sophisticated data analysis to ensure efficacy and safety at scale.
The future of obesity treatment is being written now. With the right tools, we can ensure that future arrives as quickly and safely as possible for the millions waiting for these treatments.
Learn how Lifebit powers real-time evidence and analytics for pharmacovigilance and find how our platform helps transform promising trial data into the next generation of approved therapies.

