How AI is Teaching Old Drugs New Tricks
Why the $2.6 Billion Drug Development Problem Demands a New Approach
Drug development AI is revolutionizing how pharmaceutical companies find, design, and bring new therapies to market. Here’s what you need to know:
What Drug Development AI Does:
- Accelerates target identification from years to months using machine learning on multi-omic datasets
- Predicts drug efficacy and toxicity before expensive clinical trials, reducing failure rates
- Designs novel molecules through generative AI trained on billions of chemical structures
- Optimizes clinical trials by identifying ideal patient cohorts from real-world data
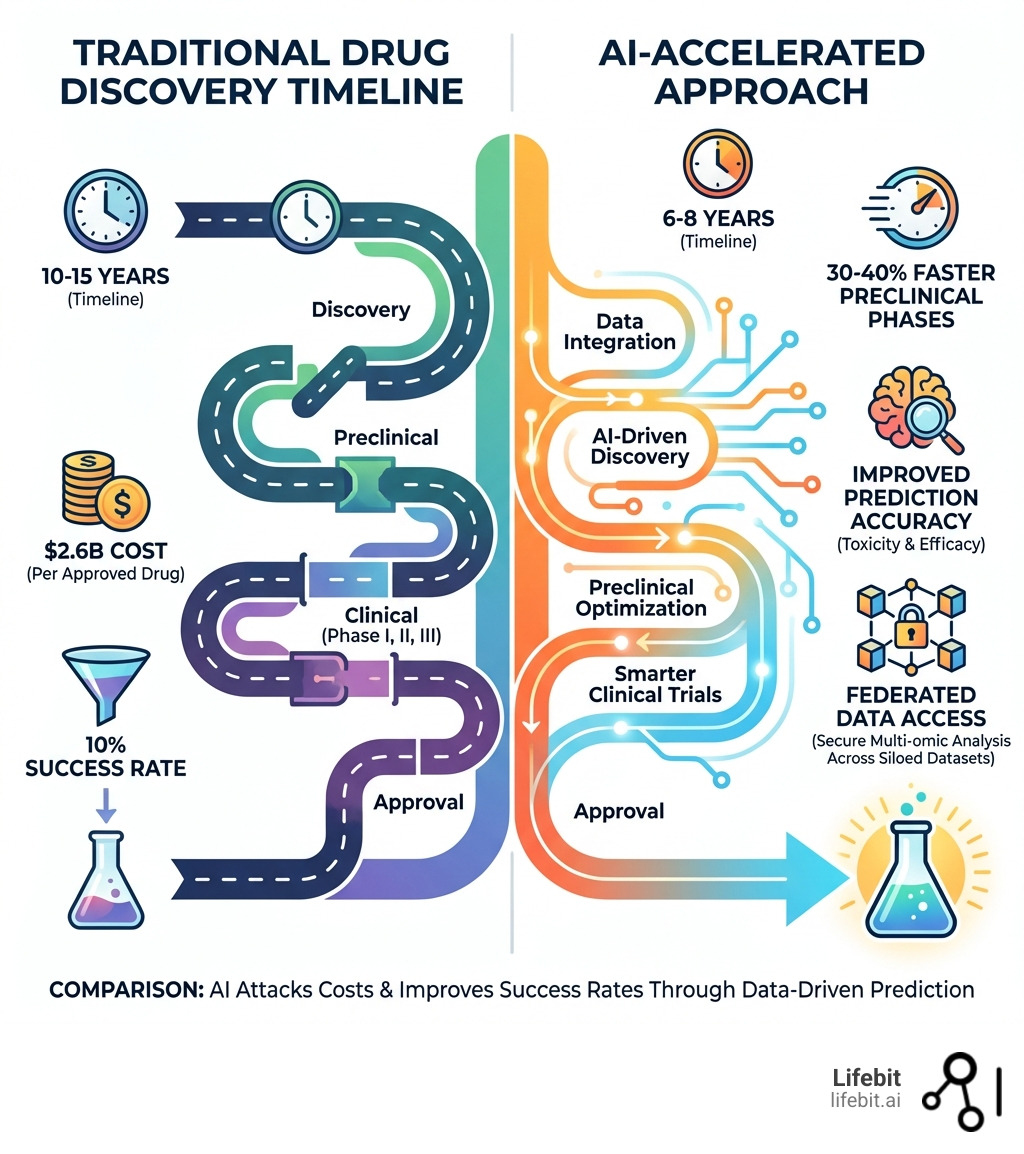
- Compresses preclinical timelines by 30-40% compared to traditional methods
The Problem It Solves:
Traditional drug discovery costs an average of $2.6 billion per approved drug and takes over a decade. Only 1 in 10 drugs entering Phase I trials ever reach patients. AI attacks both problems simultaneously—slashing costs and improving success rates through data-driven prediction.
The pharmaceutical industry has reached a breaking point. Despite massive R&D investments, productivity continues to decline. The FDA has reviewed over 500 submissions with AI components since 2016, signaling a fundamental shift in how regulators view machine learning in drug development. Yet most companies struggle to access the diverse, siloed datasets—spanning genomics, electronic health records, and chemical libraries—needed to train accurate AI models.
I’m Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent over a decade building federated AI infrastructure that lets pharmaceutical companies, regulators, and public health institutions analyze sensitive biomedical data without moving it. My background in computational biology and genomics has shown me how Drug development AI succeeds or fails based on data access, quality, and governance—not just algorithms.
Key Drug development AI vocabulary:
- biotech companies uk
- health data harmonization
- london biotech network
Why Traditional R&D Is Broken — And How Lifebit’s AI Fixes It
For decades, the pharmaceutical industry has relied on a “trial and error” approach that is increasingly unsustainable. According to the landmark DiMasi et al. 2016 study, the average cost to bring a single new drug to market has ballooned to $2.6 billion. When you consider that 90% of drugs fail during clinical trials, it is clear that the traditional model is no longer fit for purpose.
This high failure rate often stems from a lack of early-stage predictability. We spend years and billions of dollars only to find in Phase II or III that a molecule is either toxic or simply doesn’t work in humans. This is where Drug development AI changes the game. By leveraging machine learning (ML), we can move from a reactive model to a predictive one.
In the context of drug discovery and development, AI and ML are not just “fancy computers.” They are systems capable of perceiving vast environments of biological data, abstracting that information into complex models, and inferring which chemical structures are most likely to bind to a specific disease target. Scientific research on AI in drug discovery and development highlights that these tools can identify patterns in multi-omic data that are far too complex for the human brain to process alone.
At Lifebit, we address the biggest bottleneck in this process: data silos. Most high-quality biomedical data is locked behind the firewalls of hospitals and research institutes. Our federated AI approach allows researchers to bring their models to the data, rather than trying to move petabytes of sensitive information. This ensures that Drug development AI is trained on diverse, real-world populations, which is the only way to truly improve that 1-in-10 success rate.

Core Applications: From Target Discovery to Clinical Optimization
The applications of Drug development AI span the entire lifecycle of a drug, offering benefits that were unimaginable a decade ago. It is no longer just about finding a “hit” molecule; it is about end-to-end drug discovery optimization.
- Target Identification: AI can scan thousands of scientific papers, genomic datasets, and clinical trial results to identify proteins or genes that drive a specific disease. This scientific research on AI-powered therapeutic target discovery shows that ML can prioritize targets with a much higher likelihood of clinical success.
- Novel Molecule Design: Generative AI models can “dream up” entirely new chemical entities that have never existed in nature, specifically designed to fit into a target protein’s pocket.
- Optimizing Clinical Trials: By using target identification with real-world evidence, we can identify which patients are most likely to respond to a treatment, reducing trial size and duration.
Scientific research on deep learning for drug discovery has demonstrated that deep neural networks can predict the biological activity of compounds with remarkable precision. By integrating multi-omic data—genomics, proteomics, and more—Lifebit’s platform provides the “ground truth” needed for these models to thrive.
Accelerating Drug Development AI in Preclinical Phases
The preclinical phase is often where the most time is lost. Researchers must steer a labyrinth of phenomics (how cells look), transcriptomics (how genes are expressed), and proteomics (how proteins behave). Traditionally, this required labor-intensive wet lab experiments.
Today, leading-edge companies are using automated wet labs that use robotics and computer vision to capture millions of cell experiments per week. This generates massive datasets—sometimes exceeding 65 petabytes—that serve as the fuel for Drug development AI.
By using AI-driven drug discovery, we can analyze these experiments in real-time. For example, AI for biomarker discovery allows us to find molecular signatures that indicate whether a drug is working long before it ever reaches a human subject. Lifebit’s federated data access is critical here, as it allows biopharma companies to securely collaborate with academic centers to access these specialized preclinical datasets.
Predicting Toxicity and Efficacy with High Precision
One of the most transformative aspects of Drug development AI is its ability to predict ADME (Absorption, Distribution, Metabolism, and Excretion) and toxicity profiles. This is often referred to as in silico testing.
| Feature | Traditional Testing | AI-Based Models |
|---|---|---|
| Speed | Months to years | Minutes to days |
| Cost | Millions of dollars | Fraction of the cost |
| Accuracy | Dependent on animal models | High (based on human data) |
| Ethics | High animal usage | Reduced animal testing |
Scientific research on machine learning for toxicity estimation shows that ML models can now estimate the toxicity of drug candidates with accuracy levels that often surpass traditional methods. By screening molecules in “chemical space” before they are even synthesized, we can “fail fast” and focus only on the most promising candidates. Lifebit’s drug-target identification secure data solutions ensure that this predictive modeling happens in a secure, compliant environment, protecting intellectual property and patient privacy simultaneously.
The Three Pillars of Successful Drug Development AI
To move beyond the hype and deliver real clinical results, Drug development AI requires three foundational elements:
- High-Quality Data: As the saying goes, “garbage in, garbage out.” AI is only as good as the data it is trained on. This is why health data harmonization is so vital. We need curated, annotated datasets that reflect the complexity of human biology.
- Advanced Models: We are seeing a shift from simple regression models to sophisticated architectures like Generative Adversarial Networks (GANs) and Transformers. Scientific research on deep learning for drug design highlights how these models can explore the vast “chemical space” to find optimized leads.
- Robust Compute Infrastructure: Processing petabytes of multi-omic data requires specialized hardware. Modern biopharma companies are increasingly partnering with tech giants to build supercomputers custom specifically for AI workloads.
Lifebit’s AI drug discovery platform integrates these three pillars into a single, scalable environment. Our Trusted Research Environment (TRE) provides the compute power and secure data access needed to run the most advanced models without compromising on security or compliance.
Solving the “Black Box” Problem: Ethics and Explainability
As we integrate AI deeper into the fda drug approval process, we face a significant challenge: explainability. If an AI model predicts that a drug will be toxic, but cannot explain why, regulators and clinicians are unlikely to trust it. This is often called the “Black Box” problem.
Ethical considerations are also at the forefront. Algorithmic bias can occur if a model is trained on data from only one demographic, leading to drugs that are less effective for minority populations. Furthermore, data privacy is paramount when dealing with sensitive genomic information.
To address these, the industry is moving toward Explainable AI (XAI). According to scientific research on XAI for responsible AI, XAI techniques allow researchers to peek inside the model and understand which biological features led to a specific prediction. This creates a “human-AI symbiosis,” where the AI provides the insights and the pharmaceutical scientist provides the critical validation.
At Lifebit, we believe the solution to many of these ethical problems lies in breaking down data silos through federated AI. By accessing data from 5 continents, we ensure that Drug development AI is trained on a truly global and representative dataset, significantly reducing the risk of bias.
Navigating the FDA’s Evolving Regulatory Landscape
The regulatory environment for Drug development AI is changing rapidly. The FDA’s Center for Drug Evaluation and Research (CDER) has seen a massive influx of AI-related submissions. In fact, CDER has reviewed over 500 submissions with AI components between 2016 and 2023.
In early 2025, the FDA issued a pivotal draft guidance titled, “Considerations for the Use of Artificial Intelligence to Support Regulatory Decision-Making for Drug and Biological Products.” This framework emphasizes:
- A risk-based approach to AI oversight.
- Transparency in how models are developed and validated.
- Lifecycle maintenance plans to ensure AI models remain accurate over time.
We recently participated in an FDA-CTTI workshop on AI in drug development, where the focus was on how AI can support regulatory decision-making while ensuring patient safety. Lifebit’s platform is designed to support these regulatory requirements by providing a fully auditable and compliant environment for AI-powered drug discovery.
The Future of Drug Development AI
The future of the industry depends on a new generation of “bilingual” professionals—those who understand both molecular biology and data science. Specialized education programs, such as the University of Maryland MS in AI for Drug Development, are springing up to meet this demand.
We see a future where AI-powered drug development 2025 revolution is no longer a buzzword but a standard operating procedure. This will involve:
- Interdisciplinary collaboration between AI researchers and pharmaceutical scientists.
- Self-driving labs that autonomously synthesize and test new compounds.
- Digital twins of patients to simulate clinical trial outcomes before they happen.
Frequently Asked Questions about Drug Development AI
How does AI reduce the cost of drug discovery?
AI reduces costs by predicting failures early in the process (preclinical phases), which prevents the massive “sunk costs” associated with failed Phase II and III clinical trials. It also automates labor-intensive tasks like target identification and molecule design, compressing timelines by 30-40%.
What are the main challenges in integrating AI into biopharma?
The primary challenges are poor data quality and the existence of data silos. Additionally, the “black box” nature of some AI models makes it difficult to gain regulatory approval and clinician trust. Finally, there is a significant talent gap for professionals who understand both AI and drug development.
Can AI predict if a drug will fail in clinical trials?
While no system is 100% accurate, Drug development AI is significantly better at predicting failure than traditional methods. By analyzing real-world data and multi-omic signatures, AI can identify toxicity risks and efficacy gaps that often go unnoticed in animal models.
Conclusion
The era of slow, $2.6 billion drug development is coming to an end. By embracing Drug development AI, we can finally tackle the industry’s productivity crisis and bring life-saving therapies to patients faster and more affordably.
However, the success of AI in this field is not guaranteed by algorithms alone. It requires a commitment to high-quality, harmonized data and a secure, federated approach to analysis. At Lifebit, we are proud to provide the infrastructure that makes this possible, enabling biopharma companies to securely access the world’s most valuable biomedical data.
The future of medicine is data-driven, and we are just getting started. Learn more about Lifebit’s federated AI solutions and join us in the drug discovery 2.0 revolution.

