From Consent to Outcomes: Mastering Patient Data in Clinical Research

28.5M Records Breached: Fix Patient Consent Now to Win Back Trust and Unlock Research
Patient consent management is the system for handling patient approvals for treatments, procedures, and the use of their health data for research. It’s about giving patients control.
Why is this critical?
- Empowers Patients: Gives individuals control over who accesses their health information.
- Ensures Compliance: Meets strict privacy laws like HIPAA and GDPR.
- Builds Trust: Shows respect for patient privacy through transparent practices.
- Enables Research: Facilitates ethical data sharing for medical breakthroughs.
- Reduces Risk: Minimizes data breaches and legal penalties.
In healthcare, trust is collapsing. In the second half of 2022, breached patient records affected 28.5 million people. A recent survey revealed 55% of patients have lost trust in their provider, and 80% are unlikely to return after a breach.
Patients feel powerless over their medical records. Traditional paper-based consent is confusing, static, and hard to change. This reluctance to share data stalls medical science. The challenge for clinical research leaders is clear: how do we empower patients, protect their data, and open up the power of health insights?
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With 15 years in computational biology, I’ve focused on changing healthcare through innovative patient consent management and federated data analysis. This article will show you how to rebuild trust and master patient data in clinical research.
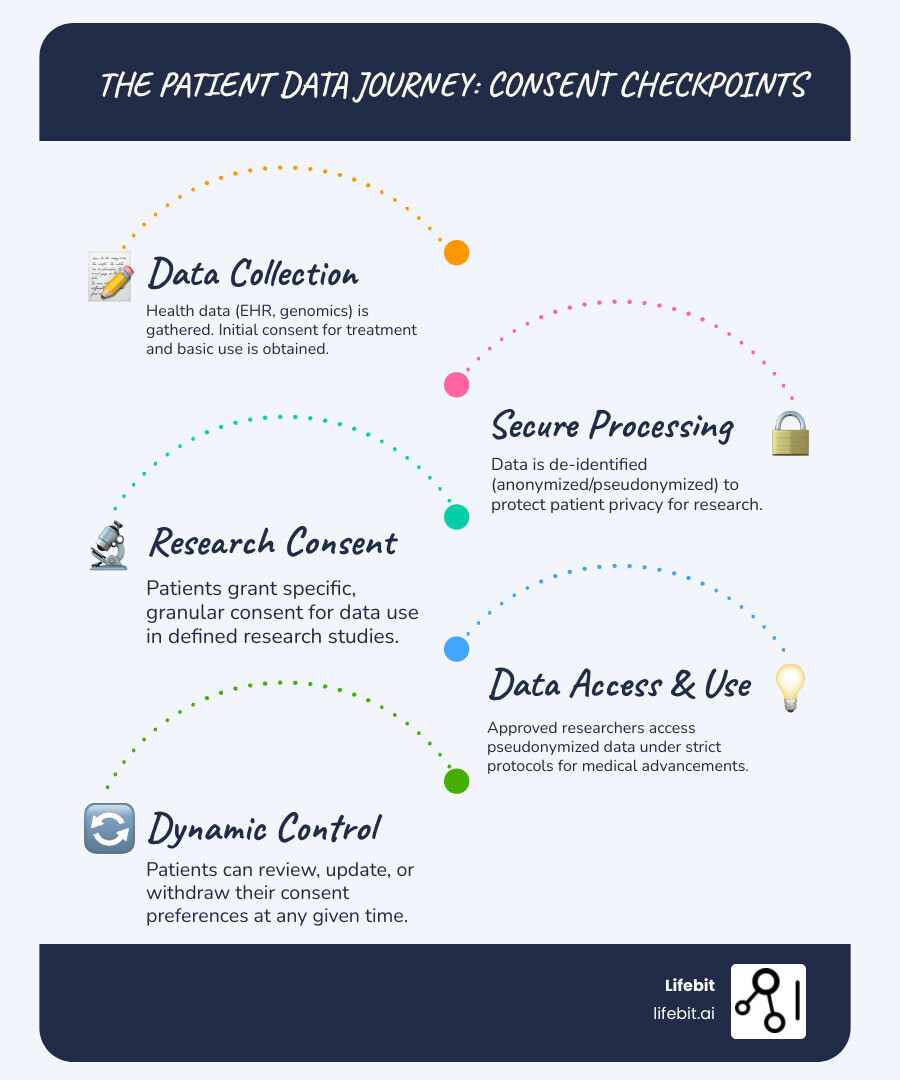
Simple guide to patient consent management:
98.6% of Hospitals Leak Data: Replace Paper Consent or Lose Patients—and Pay Fines
We have a serious problem. In the latter half of 2022, 28.5 million patient records were breached—a 35% increase from the previous year. The damage is profound. An AMA survey of 1,000 patients revealed that 55% had lost trust in their healthcare provider, and 80% wouldn’t return after such an incident.
The digital world makes it worse. A 2022 study found that 98.6% of hospital websites use third-party tracking software, such as Meta Pixel and Google Analytics. This code quietly shares patient data—including IP addresses, appointment details, and sensitive search terms related to conditions and treatments—with tech companies, social media giants, and data brokers, often without explicit patient knowledge or consent. When sensitive health information is passed around for advertising and analytics, is it any wonder trust has eroded? This crisis creates a massive barrier to medical research, which depends entirely on patients willingly sharing their data.

The Pitfalls of Paper and Siloed Systems
Our current patient consent management system is a mess. With each new provider, patients fill out more paper forms, scattering their medical history across disconnected filing cabinets and incompatible digital systems.
- Manual processes are not just slow and expensive; they are dangerously error-prone. A misplaced form or a data entry mistake can lead to a direct violation of a patient’s wishes, creating significant legal and ethical risks.
- Static consent on paper is frozen in time. Patients who want to change their preferences—for instance, to opt out of a research study they previously agreed to—face a bureaucratic nightmare of phone calls and paperwork. This leaves them feeling trapped and without control over their own information.
- Inconsistent state policies create a compliance nightmare. Rules for data sharing change dramatically from state to state. Some, like Texas, use an “opt-in” model where data sharing requires explicit permission. Others use an “opt-out” model where consent is assumed unless a patient actively objects. This patchwork makes multi-state research and operations incredibly complex and risky.
- Data silos and information blocking make interoperability nearly impossible. Fearing steep HIPAA penalties for improper data disclosure, many organizations deliberately restrict data exchange. This practice, known as “information blocking,” locks away valuable health information that could fuel life-saving research, creating a chilling effect on scientific collaboration.
Key Regulations Impacting Patient Consent Management
Navigating the regulatory landscape for patient consent management is a high-wire act, demanding a deep understanding of several overlapping and sometimes conflicting laws.
HIPAA (Health Insurance Portability and Accountability Act) sets the U.S. baseline. It allows data sharing for Treatment, Payment, and Operations (TPO) without specific, granular consent. However, its broad definitions of what constitutes TPO can lead to over-sharing of sensitive information. While the “minimum necessary” standard requires organizations to limit data use to what’s needed for a task, its interpretation varies, creating ambiguity and risk.
GDPR (General Data Protection Regulation) in Europe is far stricter. It mandates that consent must be a “freely given, specific, informed, and unambiguous indication of the data subject’s wishes.” This means broad, bundled consent is invalid. Consent must be granular, tied to a specific purpose, and as easy to withdraw as it is to give. It also grants the “right to erasure,” a key challenge for immutable data systems.
The 21st Century Cures Act was designed to combat information blocking and promote data liquidity for research and patient access. However, its penalties for blocking data are often less severe than the multi-million dollar fines for a HIPAA violation. As a result, many risk-averse organizations still choose to restrict data sharing, prioritizing penalty avoidance over the Act’s interoperability goals.
Finally, a growing number of state privacy laws—including the California Consumer Privacy Act (CCPA) as amended by the CPRA, Colorado’s CPA, Connecticut’s CTDPA, Utah’s UCPA, and Virginia’s VCDPA—are adding more layers of compliance, often modeled on GDPR’s principles. For national healthcare systems, this creates a dizzying web of obligations. The financial stakes are high, with HIPAA violations costing millions. But the real cost is the loss of trust that underpins all of healthcare and research.
Turn Consent Into Code: Blockchain Slashes Breach Risk and Speeds Data Access
The cracks in traditional consent systems are impossible to ignore. Patients feel powerless, organizations struggle with compliance, and researchers face data roadblocks. It’s time for a fundamental shift toward decentralization, particularly through blockchain technology. This isn’t just a tech upgrade; it’s a reimagining of the patient-provider relationship built on verifiable trust.
Instead of a vulnerable central filing cabinet, imagine consent decisions recorded in a shared, transparent ledger that no single entity controls. Every action is visible, permanent, and verifiable. You can update preferences instantly from anywhere, and your rules are enforced automatically by code. That’s the promise of decentralized patient consent management.
| Feature | Centralized Consent Management | Decentralized Consent Management (Blockchain-based) |
|---|---|---|
| Security | Vulnerable to single points of failure, easier for breaches. | Improved security through cryptographic hashing and distributed ledger. |
| Patient Control | Limited, often static, difficult to modify or revoke. | Granular, dynamic, real-time control over consent preferences. |
| Transparency | Opaque data flows, difficult to audit access. | Immutable audit trails, transparent logging of all consent-related actions. |
| Efficiency | Manual processes, administrative burden, data silos. | Automated via smart contracts, streamlined data sharing, reduced intermediaries. |
| Trust | Relies on trust in a single entity, prone to distrust. | Trustless environment, verifiable actions, built on cryptographic proof. |
| Interoperability | Fragmented, difficult to share across systems and organizations. | Standardized protocols, easier and more secure data exchange across ecosystems. |
How Blockchain Revolutionizes Consent
At its core, a blockchain is a shared record book that no one can alter or erase. For patient consent management, this changes everything.
- Immutability creates accountability. A consent decision recorded on a blockchain is there forever, providing irrefutable proof of what occurred and when.
- Transparency builds trust. Every attempt to access your data is logged. You can see who requested access, when, and whether your rules allowed or denied it.
- Smart contracts enforce your wishes automatically. These self-executing bits of code act as digital guardians of your preferences. For example, a patient can set a rule: “My genomic data can be used for non-commercial cancer research but not for-profit pharmaceutical development.” When a researcher queries the data, the smart contract automatically checks the researcher’s verified credentials and the query’s purpose against the patient’s rule, granting or denying access in real-time without human intervention.
- Distributed ledgers eliminate single points of failure. By distributing copies of the consent records across a network of trusted nodes (e.g., hospitals in a research consortium), the system becomes incredibly resilient to attack or failure. There is no central server to hack.
- Cryptographic hashing ensures integrity. Each block of transactions is mathematically linked to the previous one, creating a chain that is virtually impossible to forge or manipulate.
The administrative benefits are compelling. Blockchain automates burdensome paperwork, freeing staff for patient care. Research on blockchain for consent shows these systems can dramatically reduce overhead while improving compliance.
The Core Components of a Modern Patient Consent Management System
An effective system requires an ecosystem of components working together.
Patient identity management is the foundation. This goes beyond a simple username and password. Using technologies like Decentralized Identifiers (DIDs) and Verifiable Credentials (VCs), the system ensures with cryptographic certainty that you are who you claim to be before allowing any changes to consent preferences.
The consent ledger is the on-chain, immutable history of your decisions. It acts as the single source of truth, capturing what data you’ll share, why, with whom, and for how long, using pseudonymized IDs to protect your privacy.
Data access logs create a complete, on-chain audit trail. Linked to the consent ledger, this log records every attempt to access your data—whether successful or denied—providing a transparent and irrefutable history for both patients and auditors.
Purpose-based policies enable granular control. Instead of a binary “yes or no,” patients can navigate a “purpose-tree.” For example: [Main Branch: Cancer Research] -> [Sub-branch: Breast Cancer Studies] -> [Leaf: Use my genomic data but not my imaging scans]. This structure gives patients meaningful choice.
User interfaces are critical for adoption. Patients need a simple, secure portal to view and manage permissions in plain language. Researchers and clinicians need a streamlined interface to request data, where compliance checks are automated in the background, simplifying their workflow and reducing their legal burden.
Off-chain data storage is the practical solution for handling large volumes of Protected Health Information (PHI). Your actual health records (EHR data, medical images, genomic files) are too large and sensitive to store on a blockchain. Instead, they remain in secure, encrypted databases (e.g., a hospital’s data warehouse), while the blockchain stores only the immutable pointers and consent metadata. This hybrid approach offers the best of both worlds: blockchain’s security and transparency with practical, scalable data storage.
At Lifebit, we’ve built these principles into our federated platform architecture. Our approach enables secure access to global biomedical data while ensuring patient consent is always respected, automatically enforced, and transparently auditable.
62% Want a Say: Purpose-Based Consent Wins Trust and Compliance
At its heart, a truly modern patient consent management system is all about empowerment. We believe patients should be active partners in how their health data is used. A purpose-based consent model answers the call from the 62% of consumers who want healthcare providers to listen and act on their feedback. It moves beyond broad consent forms to offer granular, dynamic control directly to the patient.
Giving Patients Active Control Over Their Data
To empower patients, we must provide clear tools and simple information. A modern patient portal acts as a “command center” for health data, where patients can:
- Set granular preferences using a “purpose-tree” structure to specify what data is shared, for what purpose, with whom, and for how long.
- Exercise dynamic consent, updating or withdrawing permissions at any time, with changes instantly enforced system-wide.
- Receive notifications for data access attempts, providing unprecedented transparency.
Information must be presented in simple, accessible language, using educational materials to bridge knowledge gaps. Creating channels for open dialogue and feedback fosters a collaborative environment where trust can be rebuilt.
Balancing Privacy with the Need for Clinical Access
Patient control is paramount, but clinicians and researchers need legitimate access to data. The art of patient consent management is finding this balance.
Role-Based Access Control (RBAC) restricts data access based on a user’s professional role, ensuring they only see what’s necessary for their job. Purpose-Based Access then checks every request against the patient’s specific consent choices.
To further protect privacy, we use pseudonymization and data minimization, replacing direct identifiers and providing only the minimum data required. For secure data exchange, federated platforms are key. They allow authorized queries to run on data where it resides, returning only insights without moving the raw data itself.
Finally, emergency access protocols allow clinicians to override consent restrictions in life-threatening situations. Every override is fully audited, balancing immediate clinical needs with patient rights and full accountability.
Deploy Consent at Scale: Interoperate Fast, Pass Audits, Avoid Fines
Building a decentralized, blockchain-based patient consent management system requires weaving new technology into the complex fabric of existing healthcare IT.

The architecture must be built on three pillars: interoperability with legacy systems, scalability to handle millions of records, and robust data governance for accountability. For interoperability, the system must communicate seamlessly with existing Electronic Health Record (EHR) platforms via modern APIs and standards like HL7 and FHIR (Fast Healthcare Interoperability Resources). For scalability, a private or consortium blockchain is often used in healthcare to provide the high transaction throughput needed for enterprise applications, overcoming the performance limitations of public blockchains. Challenges like system integration, performance tuning, and change management are significant. However, the rewards—improved trust, accelerated research, and unlocked data insights—are transformative. The cost of not implementing modern patient consent management, measured in breaches, fines, and lost trust, is far greater.
Addressing the ‘Right to Erasure’ on an Immutable Blockchain
How can you delete data from a permanent blockchain, as required by regulations like GDPR’s Article 17 “right to be forgotten,”? The solution lies in a hybrid architecture that separates consent logic from personal data.
We never store sensitive patient data (PHI) on the blockchain. Instead, the actual data resides in secure, encrypted, off-chain databases. The blockchain stores only the immutable records of consent, cryptographic hashes (data fingerprints), and encrypted pointers to the off-chain data.
When a patient exercises their right to erasure:
- The encrypted pointer on the blockchain is cryptographically invalidated or “broken.” The on-chain record of the pointer’s existence remains for audit purposes—proving the deletion request was honored—but it no longer leads to any data.
- The actual off-chain data is permanently deleted from its secure storage system, fulfilling the legal requirement.
This method satisfies regulatory requirements for erasure while preserving the blockchain’s integrity as a tamper-proof record of events. It’s a compliance strategy that respects both patient rights and the technology’s core strengths.
Practical Applications in High-Stakes Research
Robust patient consent management unlocks research previously blocked by trust issues and data silos. At Lifebit, our federated AI platform is built for these high-stakes applications, enabling secure, real-time access to global biomedical data.
- Biobanking and Genomics: Donors can provide granular, dynamic consent, specifying exactly how their genetic data is used. This is crucial for genomics, as a person’s DNA is inherently identifiable and has implications for their relatives. A dynamic system allows researchers to re-contact donors for permission to use their data in future studies, a process that is cumbersome and often impossible with paper consent. This flexibility builds trust and encourages long-term participation, as demonstrated in research on blockchain for dynamic consent.
- Real-World Evidence (RWE) Studies: RWE is health information derived from sources outside of typical clinical trials, such as EHRs, insurance claims, and data from wearable devices. Our platform facilitates large-scale, compliant RWE research by providing federated analytics and transparent consent management. This allows researchers to train AI models and gather insights from real-world clinical data across multiple institutions without ever moving or centralizing the sensitive data, ensuring privacy is maintained.
- Pharmaceutical Trials: Blockchain-powered consent streamlines the entire trial process. It enhances patient recruitment and retention by offering transparency and control, which can help build trust with underrepresented communities and improve trial diversity. Furthermore, it creates an immutable, time-stamped audit trail for every consent action, which simplifies compliance and strengthens regulatory submissions to bodies like the FDA and EMA.
- Disease Registries and Collaboration: For rare diseases or public health crises, progress depends on pooling data from many institutions. A decentralized system enables secure, consented data sharing across these institutional boundaries, overcoming the common reluctance to share data. Our platform powers this collaboration with built-in data harmonization, AI/ML analytics, and federated governance, allowing researchers to find patterns in a larger, more diverse dataset while respecting the rules of each contributing organization.
By engaging patients transparently, we can unlock the full potential of health data to achieve public health goals and speed the discovery of new medicines.
Stop Guessing: Fast Answers to Patient Consent Questions
It’s natural to have questions about how your health data is used. Here are some clear answers about patient consent management.
What is the difference between consent for treatment and consent for research?
Consent for treatment is your agreement to a medical procedure intended to help you directly. The focus is on your immediate, personal well-being.
Consent for research is your permission to use your data or samples to advance general medical knowledge. While it may not benefit you directly, it helps scientists develop new treatments for everyone. Modern patient consent management systems give you detailed control over how your information contributes to science.
How can I find out who has accessed my health data?
This is key to transparency. With a modern, blockchain-based patient consent management system, every attempt to access your data is recorded on an unchangeable audit trail. Through a secure patient portal, you can see exactly who accessed your data (e.g., your doctor, a research study), when they did, and for what purpose. This openness empowers you to monitor your health information.
Can I change my consent preferences after I’ve given them?
Absolutely. This is a core feature of modern systems, known as dynamic consent. Old paper forms are static, but with an e-consent platform, you can create, update, or withdraw your consent at any time through a secure portal. If you agree to a study and later change your mind, you can easily revoke permission. The system reflects your changes instantly, giving you continuous, real-time control.
Act Now: Modern Consent Wins Trust and Accelerates Research
We’ve been on quite a journey together, haven’t we? We started by looking at the stark reality of the trust deficit in medical research. We saw how traditional, fragmented systems for patient consent management led to worrying data breaches, deep patient distrust, and ultimately, hindered the very research that could save lives. The challenges are clear: from inefficient paper processes and confusing state policies to the complex tightrope walk of HIPAA and GDPR compliance.
But here’s the good news: we don’t have to stay stuck in the past. There’s a path forward, rooted in decentralized technology. Imagine a fresh start, built on principles of openness and unwavering security. By embracing blockchain’s incredible power – its ability to create unchangeable records, provide crystal-clear transparency, and automatically enforce rules through smart contracts – we can truly transform how consent is handled. This new paradigm allows us to build robust systems with secure patient identity management, clear on-chain consent ledgers, detailed access logs, and smart, purpose-based policies, all wrapped up in user-friendly interfaces that empower everyone.
At the heart of this change is empowering you, the patient. Giving patients granular, dynamic control over their own health data isn’t just an ethical choice; it’s the very cornerstone of ethical research and accelerated findy. Imagine having your own digital command center – a portal or preference center – where you can easily decide exactly how your data is used, and change your mind whenever you want. This patient-first approach, combined with smart controls like Role-Based Access Control (RBAC) and pseudonymization to protect privacy, ensures that critical clinical research can still thrive. Even tricky issues like the “right to erasure” can be handled thoughtfully by separating the sensitive data from the unchangeable consent records.
The real magic happens in high-stakes research. With robust patient consent management in place, doors open to incredible possibilities. Think about accelerating findies in areas like biobanking and genomics, gaining valuable insights from Real-World Evidence (RWE) studies, or speeding up new drug development in pharmaceutical trials. It even makes vital cross-institutional collaboration and the creation of disease registries much smoother and more secure.
At Lifebit, we believe that patient empowerment isn’t just a buzzword; it’s the cornerstone of ethical research and faster findies. Our federated AI platform brings these principles to life. It’s designed to enable secure, compliant research across vast global datasets, all without ever compromising patient privacy or control. We’re dedicated to helping build a future where trust isn’t just hoped for, but built in, where data is managed with integrity, and medical breakthroughs happen faster, always respecting the fundamental rights of every patient.
Ready to see how?
Learn how to enable secure, compliant research across global datasets with Lifebit’s platform.
Explore more about Lifebit’s solutions for data-driven healthcare.

